You could feel the energy and excitement at the 2022 HIMSS Global Health Conference & Exhibition (HIMSS22) held this ...
April 29, 2022 — Bristol Myers Squibb announced that the U.S. Food and Drug Administration (FDA) approved Camzyos ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
During a Philips international media roundtable at HIMSS22, Mandi Bishop, vice president analyst at Gartner, a ...
April 28, 2022 — The U.S. Food and Drug Administration (FDA) is alerting healthcare providers to the possibility that ...
April 28, 2022 — Aspirin therapy, as opposed to statin use, for non-obstructive coronary artery disease does not reduce ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
April 28, 2022 — Today, the Department of Health and Human Services (HHS), through the Centers for Medicare & Medicaid ...
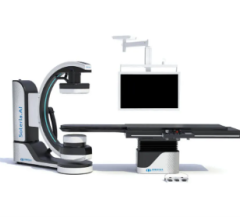
April 27, 2022 – Omega Medical Imaging has announced the release of the revolutionary Soteria.AI. Designed specifically ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
April 27, 2022 — The U.S. Food and Drug Administration (FDA) has issued a Class I recall for the Medtronic Harmony ...
April 27, 2022 – CathVision, a medical technology company developing innovative electrophysiology solutions designed to ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
April 25, 2022 – There’s no safe way to get a close-up view of the human heart as it goes about its work: you can’t just ...
April 25, 2022 — Pfizer is voluntarily recalling five (5) lots of Accupril (Quinapril HCl) tablets distributed by Pfizer ...


 April 29, 2022
April 29, 2022