November 27, 2011 – Siemens Healthcare has launched five new mobile solutions that help provide imaging specialists and referring physicians with secure access to diagnostic images and reports, both inside and outside the hospital. syngo.via WebViewer enables fast viewing of images with stunning graphics and syngo.via WebReport provides secure access to reports and images anywhere. Both syngo.via WebViewer and syngo.via WebReport are available for download from the App Store and are designed to operate on the iPad, iPhone and iPod Touch. In addition to these advanced visualization solutions, Siemens has unveiled syngo Workflow Mobile, which delivers radiology information system (RIS) capabilities for technologists and radiologists to a variety of mobile devices and syngo Dynamics Mobile, which allows cardiovascular imaging specialists to extend their access to image and report review beyond the workstation to Internet-enabled devices; and syngo.plaza virtualized, which brings the acclaimed functionality and user interface of syngo.plaza to PC or Apple computers and mobile devices, even those operating on lower bandwidth networks such as 3G.
November 27, 2011 — Vital, a Toshiba Medical Systems Group Company and provider of advanced visualization and analysis solutions for healthcare providers, will represent the Healthcare and Imaging Informatics business unit of Toshiba Corporation (Toshiba) at the 97th Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA) in Chicago (booth 8318, North Hall). The company will also release Version 6.2 of its Vitrea Enterprise Suite (VES) and VitreaView solutions, which include clinical and usability enhancements.
November 23 2011 – At the 2011 VEITH symposium, Arno von Ristow, M.D., department of vascular and endovascular surgery, Centervasc-Rio & Clinica Sorocaba, Catholic University of Rio de Janeiro, Brazil, presented some strong findings regarding the use of endovascular techniques to deal with the management of para-anastomotic aneurysms.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Biodex is introducing its new line of vascular ultrasound tables for vascular and echocardiography imaging during RSNA 2011.
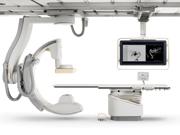
Over the last 10 years, hospitals have phased out analog image intensifiers for flat-panel, digital detector angiography systems in their cath labs. As hospitals look to purchase current-generation digital angiography systems, buyers should be aware of several new technologies.
We are at an inflection point with many aspects of medicine, not the least of which concerns choosing the correct tool to discover and manage coronary artery disease. The American landscape of clinical and imaging service providers is going through a major reshuffle. Coronary computed tomography angiography (CTA) is growing in utilization, taking its place as an established modality with AHA-, ACC- and ACR society-derived appropriate use standards. However, this growth in CT utilization and the resultant growth in per capita radiation exposure have elevated a very public debate about the risk of causing cancer versus the value of its information content.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
November 23, 2011 – Vascular occlusions are blocked blood vessels caused by a blood clot in an artery. Blocks in the femoral artery are particularly problematic, and vascular surgeons continue to research and debate on the best methods to treat these clots. Results from the THRUPASS Study Group’s randomized controlled trial comparing superficial femoral artery (SFA) lesion treatment with either endoluminal polytetrafluoroethylene (PTFE) thrupass (Viabahn) or with surgical PTFE bypass were presented to attendees at the 38th Annual VEITH symposium in New York City by Mauri J.A. Lepantalo, M.D., Helsinki University Central Hospital, Helsinki, Finland.
November 23, 2011 – Many specialists question whether a permanent inferior vena cava (IVC) filter can be developed where it does not have to be retrieved from the patient.
November 23, 2011 – Medical technology incubator Therix Medical announced the launch of spin-off Bluegrass Vascular Technologies, the flagship product of which is the Surfacer Inside-Out Access catheter system, a proprietary instrumentation set that allows physicians to perform a novel “inside-out” approach to gain venous access.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
November 23, 2011 – The U.S. Food and Drug Administration (FDA) allowed marketing of the first system that can repair a failed or problematic aortic endograft, a fabric tube used to repair a dangerously large aortic aneurysm, a bulge in the large blood vessel that carries blood away from the heart.
November 23, 2011 –Varian Medical Systems will exhibit its PaxScan digital image detectors and new PaxPower X-ray tubes at the MEDICA 2011 International Trade Fair in Dusseldorf, Germany from November 16-19, 2011.

When the final space shuttle mission launched July 8, 2011, a cardiovascular ultrasound (echocardiography) system was included in its payload, destined for the International Space Station (ISS). The National Aeronautics and Space Administration (NASA) selected GE Healthcare’s Vivid q echo system following a rigorous regimen of spaceflight hardware qualification and acceptance testing.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
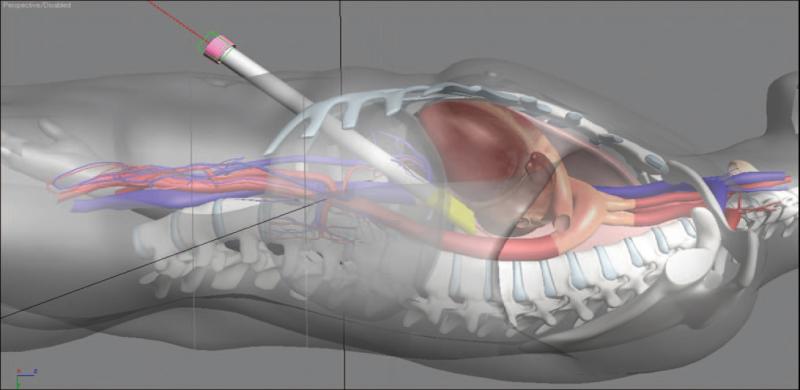
The ideal treatment of AF would be a truly minimally invasive procedure with results comparable to the Cox maze. This goal led to the convergence of surgical and electrophysiologic expertise to create a hybrid procedure for the treatment of symptomatic persistent AF.Using a pericardioscope, which is passed transabdominally through the central tendon of the diaphragm, the surgeon is able to directly visualize both atria, including the posterior left atrium and both sets of pulmonary vein.

Within the United States, the anticipated approval and launch of TAVR has received considerable attention, with speculation about who will perform the procedure. For example, many physicians are advocating a need for cardiac surgeons and interventional cardiologists to collaborate and work together as this technology becomes available in the United States.
In the past, when a patient suffered a prolonged cardiac or circulatory arrest during percutaneous coronary intervention (PCI) in the catheterization laboratory (cath lab) at Skane University Hospital–Lund, it was a catastrophic event. As any interventional cardiologist knows, the mortality rates in these situations are very high because it is essentially impossible to perform effective manual chest compressions while continuing PCI, especially in cases where prolonged resuscitation is required.

 November 27, 2011
November 27, 2011







