
May 11, 2012 — Modern cardiac catheterization laboratories bear scant resemblance to the cath labs of a decade ago. An updated consensus statement released this week offers physicians guidance on how to excel in this new diagnostic and therapeutic milieu, with specific recommendations on setting up, operating and maintaining the highest standards of quality in a contemporary cardiac cath lab.
May 11, 2012 — The Sorin Group is launching new implantable devices with a proprietary algorithm system as well as three leads, and releasing data showing improved patient outcomes with a novel CRT (cardiac resynchronization therapy) optimization system. The company highlighted this news during the Heart Rhythm Society 2012 meeting this week in Boston.
May 11, 2012 — St. Jude Medical Inc. announced U.S. Food and Drug Administration (FDA) approval of its Assura portfolio of implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-Ds). The new implantable defibrillators feature SecureSense RV Lead Noise Discrimination, an algorithm that expands the St. Jude Medical ShockGuard technology.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
May 11, 2012 — A team of scientists led by Stanley Fricke, M.D., of the Children's National Medical Center in Washington, D.C., broke the "magnetic resonance imaging (MRI) sound barrier," a finding that could lead to a hundred-fold increase in MRI speed, according to a new clinical study published this week in the peer-reviewed journal Medical Physics.
May 10, 2012 — New research confirms thrombus aspiration (TA) during percutaneous coronary intervention (PCI) in patients with acute ST-segment elevation myocardial infarction (STEMI) provides long-term outcomes similar to conventional intervention with bare-metal or drug-eluting stents.
May 10, 2012 — CompuMed, Inc. announced it will offer GE Healthcare's compact ECG platform, the MAC 800, as part of a next-generation telemedicine enhanced electrocardiogram (ECG) system.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
May 9, 2012 — Sound Interventions Inc. announced the successful completion of the company's first-in-human clinical trial to treat resistant hypertension, SOUND-ITV.
May 9, 2012 — Boston Scientific Corp. announced U.S. Food and Drug Administration (FDA) approval and market launch of its Ingenio and Advantio pacemakers and Invive cardiac resynchronization therapy pacemakers (CRT-P).
May 9, 2012 – The College of Healthcare Information Management Executives (CHIME) submitted its comments on the proposed rules for Stage 2 Meaningful Use (MU), calling for more time to allow healthcare organizations to better prepare.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
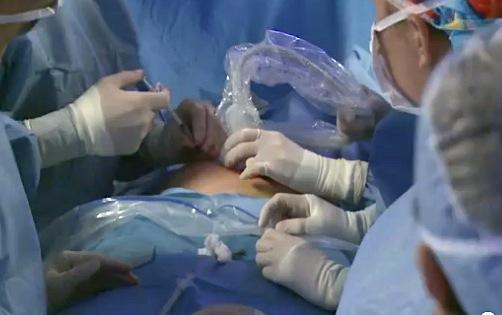
May 8, 2012 – The team at Texas Children's Fetal Center has successfully completed two in-utero fetal cardiac interventions to treat hypoplastic left heart syndrome (HLHS), a congenital heart defect that is one of the most complex heart defects to treat.
May 7, 2012 — Wide Beam Reconstruction (WBR) reduces the required radiopharmaceutical dose and image acquisition time by 50 percent for diagnostic quality myocardial perfusion imaging (MPI) single photon emission computed tomography (SPECT), according to a new study published in the March/April 2011 issue of the Journal of Nuclear Cardiology.
May 7, 2012 - On May 4, the American College of Radiology (ACR) submitted comments to the Centers for Medicare and Medicaid Services (CMS) and the Office of the National Coordinator for HIT (ONC) regarding the agencies' March 7, 2012, proposed rules to revise and update the professional and technology requirements of the EHR Incentive Program (“meaningful use”). The ACR IT and Informatics Committee (ITIC) - Government Relations Subcommittee compiled the comments with feedback from ACR members, allied organizations and other stakeholders.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
May 4, 2012 - CardioDex announces initial commercial use of a new femoral access site closure device. Recent data from commercial use of the device on 41 patients at St. Marien Hospital in Siegen, Germany showed 100% success rate and no complications.
May 4, 2012 - Edwards Lifesciences Corp. announced that new data from the European multi-center Triton trial studying the Edwards Intuity valve system highlights the promise of several important benefits, including the facilitation of small-incision surgery for aortic valve replacement (AVR), a high procedural success rate, and consistent and sustained hemodynamic valve performance at one year. The data were presented today during the Emerging Technologies and Techniques Forum at the American Association for Thoracic Surgery's 92nd annual meeting in San Francisco.
May 4, 2012 - Angioslide Ltd., a provider of embolic capture angioplasty solutions, announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its new 3x100 mm Proteus device for treating peripheral artery disease in below-the-knee (BTK) vasculature. The new 3/100 mm device accommodates 0.014-inch guidewires, which significantly broaden the potential use of Proteus technology.

 May 11, 2012
May 11, 2012










