
In a study designed to see if doctors who are told the exact price of expensive medical tests like magnetic resonance imaging (MRI) in advance would order fewer of them, Johns Hopkins researchers got their answer: No.

According to Millennium Research Group (MRG), increasing awareness and diagnosis of peripheral vascular disease (PVD) along with the introduction of improved next-generation devices will lead the United States peripheral vascular (PV) device market to grow strongly to reach $3.3 billion by 2017.
AngioScore Inc. announced the launch of its new 100 mm length AngioSculpt PTA Scoring Balloon Catheters for the treatment of peripheral artery disease (PAD) below the knee (BTK).
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Fujifilm Medical Systems U.S.A. Inc. brought a broad portfolio of diagnostic and informatic solutions to the 2012 meeting of the Radiological Society of North America (RSNA). The company showcased its advanced Cardiovascular product, Synapse Cardiovascular 5.0.
January 25, 2013 — Mobile drug reference app provider Epocrates recently conducted its annual survey of cardiologists to uncover the biggest trends and challenges facing them today. The survey explores their perspectives on a range of issues related to patient care, healthcare technology usage/adoption and practice concerns. Following are some highlights from the survey findings.
The American College of Cardiology (ACC) has released the late-breaking clinical trials that will be highlighted as the cutting edge of cardiology at the ACC.13 annual meeting March 9-11 in San Francisco.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
BRIT Systems announced new capabilities for their digital imaging and communications in medicine (DICOM) routers that can pre-fetch studies from multiple servers and/or facilities based on select criteria that do not need to include the patient ID. The router morphs the patient ID in a pre-fetched study into the original or trigger ID, storing the pre-fetched IDs into alternate ID fields in both the database and the DICOM header. The new morphing router facilitates the creation of a single patient record and enables clinicians to view all studies on a single timeline. With this capability, clinicians will have access to a complete, cross-facility patient record that can be used to reduce unnecessary or duplicate imaging studies. The new capabilities were highlighted at the Radiological Society of North America (RSNA) annual meeting.
AirStrip Technologies Inc. announced that it has entered into a long-term strategic partnership with Vanguard Health Systems Inc.
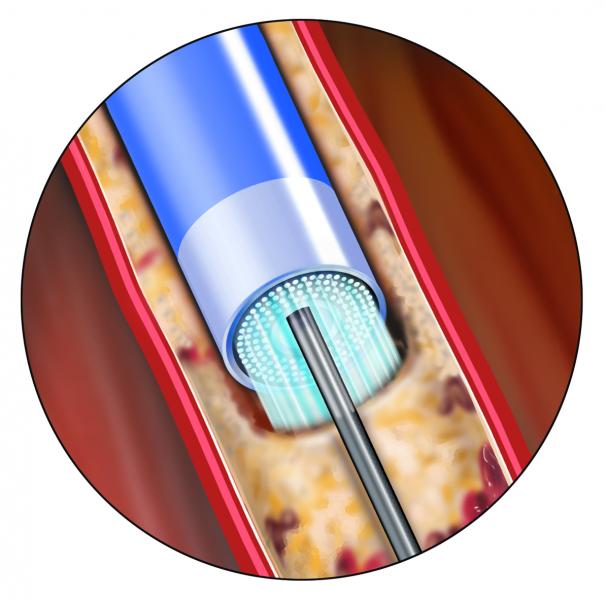
The Spectranetics Corporation announced final results from the PATENT (Photo-Ablation using the TURBO-Booster(R) and Excimer Laser for In-Stent Restenosis Treatment) study evaluating the safety and efficacy of the Spectranetics peripheral laser atherectomy catheters for the treatment of in-stent restenosis (ISR) in the femoropopliteal artery in the leg. ISR occurs as a result of narrowing of the artery in patients who have undergone stenting for the treatment of peripheral arterial disease (PAD). There is currently no U.S. Food and Drug Administration (FDA)-cleared or approved device to treat peripheral in-stent restenosis, which remains a major unsolved medical problem.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
According to Millennium Research Group (MRG), the segment of the U.S. nonvascular interventional radiology device market with the fastest growth and greatest potential is ablation devices, comprising radiofrequency (RF) ablation, cryoablation and microwave ablation devices. The overall market will grow moderately to $295 million by 2017.
Bathing dangerous blood clots in special medication, breaking them up with jets of saline and vacuuming them out of the body is a fast, effective method of treating deep vein thrombosis (DVT), suggests data being presented at the 25th annual International Symposium on Endovascular Therapy (ISET).
St. Jude Medical Inc. announced European CE mark approval of its ViewFlex Xtra Intracardiac Echocardiography (ICE) Catheter. Designed for control and maneuverability during complex cardiac ablation procedures, the technology will be on display at the eighteenth annual Boston AF Symposium.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Peri-natal systems real-time clinical decision support company PeriGen Inc. announced the results of an independent validation study on fetal ECG strip interpretation favorably comparing PeriGen PeriCALM Patterns software with the findings of three experts from the National Institute of Health (NIH). The U.S. Food and Drug Administration (FDA)-cleared fetal strip interpretation technology is a component of the company's perinatal central surveillance offering.

Using tiny balloons to temporarily block blood flow to the uterus during a high-risk Caesarean-section delivery can save the life of the mother while preventing hysterectomy and preserving fertility, suggests research being presented at the 25th annual International Symposium on Endovascular Therapy (ISET).
Cordis Corporation announced the presentation of the two-year STROLL study results at the Abstracts and Late Breaking Clinical Trials session at ISET 2013.

 January 29, 2013
January 29, 2013









