Bayer Healthcare is expanding its global distribution network for the Medrad Intego positron emission tomography (PET) Infusion System through a new distribution agreement with Comecer SPA. Through this agreement, Bayer and Comecer are providing customers with an integrated nuclear medicine solution – combining the clinical administration capabilities of Intego with the Comecer's protection technologies for PET/CT centers around the world.
Researchers at The Johns Hopkins University and Yale University have discovered that a specialized receptor, normally found in the nose, is also in blood vessels throughout the body, sensing small molecules created by microbes that line mammalian intestines, and responding to these molecules by increasing blood pressure. The finding suggests that gut bacteria are an integral part of the body’s complex system for maintaining a stable blood pressure.

St. Jude Medical Inc. announced plans for a new landmark study that will evaluate whether renal denervation and medication can provide health benefits to patients beyond lowering high blood pressure. The EnligHTNment trial is the first large-scale study that will examine the long-term effects of renal denervation in patients who have uncontrolled hypertension to see if renal denervation also reduces the risk of major cardiovascular events such as heart attack, stroke and death.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The American College of Radiology supports the Diagnostic Imaging Services Access Protection Act (H.R. 846), recently introduced by Reps. Pete Olson (R-TX), Peter Roskam (R-IL), John Barrow (D-GA), Betty McCollum (D-MN) and 38 House cosponsors. H.R. 846 would correct a 25 percent multiple procedure payment reduction to Medicare reimbursement for interpretation of advanced diagnostic imaging scans performed on the same patient, in the same session.
To address the rising costs of healthcare, we must improve the way that health care is delivered, including coordinating care better and improving the safety of care, stated the Centers for Medicare and Medicaid Services (CMS) in its assessment of healthcare reform, released Feb. 28.
Blood pressure (BP) monitoring technology specialist SunTech Medical will unveil its new cardiac stress BP device at the American College of Cardiology 2013 Scientific Session, March 9-11 in San Francisco.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Preventice Inc. will showcase its breakthrough mobile health solutions for remote patient monitoring, patient education and patient engagement technologies at the American College of Cardiology (ACC) annual meeting, March 9-11, 2013 in San Francisco.
OrbusNeich announced that OrbusNeich Medical GmbH has commenced patent infringement actions in Germany and The Netherlands against Boston Scientific Corp. and its distribution affiliates in those countries. The complaint alleges Boston Scientific infringed two European patents covering certain novel stent designs.
Specialists at Stony Brook Medicine’s Cerebrovascular and Stroke Center (CVC) are treating patients with a new generation of blood clot removal devices that show promise in successfully revascularizing stroke patients, including those with large vessel blockages. The Solitaire Flow Restoration Device and the Trevo device, approved by Food and Drug Administration (FDA) in 2012 to treat stroke caused by the sudden obstruction of a brain blood vessel (acute ischemic stroke) showed improved results over a previous standard and first generation clot-removal device in clinical trials.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
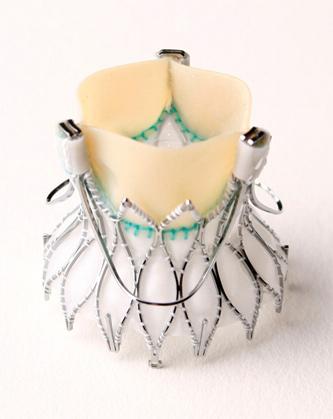
Medtronic Inc. announced European CE (Conformité Européenne) mark approval for its Engager Transcatheter Aortic Valve Implantation (TAVI) System with transapical delivery catheter. The system is indicated to treat patients with severe aortic stenosis who are at high or extreme risk for surgical aortic valve replacement (SAVR).

The Healthcare Supply Chain Association (HSCA) today launched Medical Device Tax Watch, a website dedicated to raising awareness of efforts of some medical device manufacturers to pass the costs of the medical device excise tax (MDET) to American hospitals, healthcare providers and patients. The new site will be a resource for hospitals and a tool for monitoring the device marketplace, and can be found at www.devicetaxwatch.com.
Corindus Vascular Robotics will showcase its U.S. Food and Drug Administration (FDA)-cleared CorPath 200 Vascular Robotic-Assisted System at the American College of Cardiology 2013 meeting, March 9-11.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Strain imaging for echo is increasingly being recommended to assist in assessing cancer patients at risk for heart disease. Strain Imaging can provide valuable information when monitoring patients for cardiotoxicity resulting from therapies used to treat cancer.
Schiller's new medilog AR12 plus ECG Holter recorder includes enhanced features such as P-wave detection for atrial analysis, patient movement pattern recognition by on-board three-axis accelerometers, and special purpose noise suppression circuitry optimized for electrocardiogram (ECG). The system also offers a color display,? hook-up impedance test and built-in microphone.?
The Schiller 12-Lead ECG MS-2015 unites precision, performance and a sophisticated but simplified user interface.


 March 05, 2013
March 05, 2013









