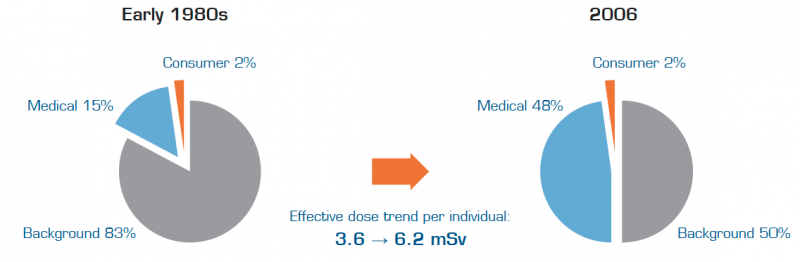
The significant increase in radiation exposure and uncertainty regarding the risk of cancer have raised concerns leading to a recent change of direction within medical imaging. Several initiatives to lower dose levels are being established and many healthcare providers have initiated dose reduction programs to guard patient safety and to comply with new regulations.

The radical increase in patient exposure to radiation from medical imaging over the last two decades has created great concerns about its inherent risks. Today, one of the highest priorities on many hospital agendas is to break this trend by achieving improved control of the radiation exposure to their patients.

Researchers say they have achieved enough coherence of the magnetic moment inherent in the defects of miniscule diamond fragments to harness their potential for precise quantum sensors in a material that is biocompatible.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Ziehm Imaging, a developer, manufacturer and supplier of mobile C-arms, will showcase the first motorized mobile C-arm for use in hybrid ORs at the Radiological Society of North America Annual Meeting (RSNA 2013) in Chicago.
Siemens Healthcare will introduce version VA30 of its routine 3-D and advanced reading software syngo.via at the Radiological Society of North America Annual Meeting (RSNA 2013).
After cardiac surgery, healthcare-associated infections (HAIs) are common complications associated with increased morbidity, mortality, and use of resources.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

Combining genetic data with clinical information to determine the initial dosage of the blood thinner warfarin, used to prevent blood clots in the circulatory system, was no more effective in achieving stable anticoagulation than using only clinical information, according to a National Institutes of Health-funded clinical trial.
Medic Vision Imaging Solutions Ltd. recently signed agreements with three major healthcare purchasing organizations, namely Premier and Health Trust PG, to offer the SafeCT to thousands of their member hospitals and other healthcare providers at pre-negotiated special terms.
Barco, a supplier of diagnostic and visualization systems, will demonstrate a wide array of imaging technologies focused on providing an integrated approach to patient care. Barco will present its latest display systems, networked digital OR solution and interactive patient care products at the Radiological Society of North America Annual Meeting (RSNA 2013) Dec. 1-5 in Chicago.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Vital Images Inc. will participate in the Image Sharing Demonstration (North Building, Hall B, booth 8140) at the annual meeting of the Radiological Society of North America (RSNA), Dec. 1-5 in Chicago.
Etiam Corp. will present the Etiam-Connect along with its Nexus 4.2 update, which features a smoother client interface, at the 2013 annual meeting of the Radiological Society of North America (RSNA).
ContextVision will debut four real-time ultrasound image enhancement packages as part of its US PlusView Family at the Radiological Society of North America Annual Meeting (RSNA 2013) in Chicago.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Barco, a supplier of diagnostic systems, presented its MDSC line of network-ready surgical displays.
Millar Inc. and CD Leycom announced the appointment of Millar as the exclusive U.S. distributor of CD Leycom’s clinical Inca in pressure-volume (PV) loop system and disposable clinical PV loop catheters.

A 320-detector computed tomography (CT) scanner that shows both anatomy within coronary arteries and blood flow can accurately sort out which people need an invasive procedure to identify coronary blockages, according to an international study.

 November 26, 2013
November 26, 2013













