Pablo Uceda, M.D., vascular surgeon with DFW Vascular, Dallas, Texas, was successful in using a new device to clear blood clots out of a patient's legs.
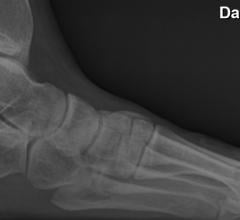
Patients suffering from chronic plantar fasciitis now have a new weapon against this debilitating foot ailment, according to research presented at the Society of Interventional Radiology's Annual Scientific Meeting.
At one time, many children born with congenital heart disease (CHD) suffered from issues that carried fatal prognoses. Thanks to technological advancements in the past 30 to 40 years, Elizabeth Adams, M.D., of Penn State Hershey Children's Heart Group, can predict that a child born today has a 90 percent chance of living to adulthood, even with severe CHD.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The U.S. Food and Drug Administration (FDA) approved the VenaSeal closure system to permanently treat varicose veins of the legs by sealing the affected superficial veins using an adhesive agent.
Point of Care Anticoagulation software (PCDS AC) optimizes delivery of anticoagulation (AC) drugs and reduces adverse events associated with anticoagulation therapy.
NDS Surgical Imaging (NDSsi) announced the release of the 27-inch Radiance Ultra, a next-generation surgical visualization platform. The ultra-high level of brightness from the LED backlight overcomes reflections and glare that occur in high ambient light environments and increases the usable contrast ratio, allowing surgeons to more easily visualize recessed anatomy.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Critical Diagnostics announced it has expanded its cardiac biomarker testing product portfolio by CE marking the Aspect-PLUS ST2 test, a rapid quantitative ST2 test. Results are reported in under 30 minutes.
A team of scientists led by Johns Hopkins cardiologist and biomedical engineer Hiroshi Ashikaga, M.D., Ph.D., has developed a mathematical model to measure and digitally map the beat-sustaining electrical flow between heart cells.

For the first time, researchers at The University of Texas Health Science Center at Houston (UTHealth) were able to enroll patients at other hospitals into an acute stroke clinical trial.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The Society of Thoracic Surgeons (STS) has released the first publicly accessible national report of surgical outcomes from its Congenital Heart Surgery Database (CHSD), a component of the STS National Database. Public reporting results are available at www.sts.org/publicreporting.

Key data from many transcatheter aortic valve replacement (TAVR) clinical trials will be presented as late-breakers at the American College of Cardiology (ACC) Scientific Session this weekend, March 14-16, 2015, in San Diego, Calif. While the three-day conference will be packed with lectures, poster presentations, case studies, etc., most investors will be waiting for TAVR-related clinical data from Edwards and Medtronic. We expect these data to be positive for both vendors, which will only help to solidify their hold on the global TAVR market.
The first annual financial results of Xofigo (radium-223 dichloride) from Bayer are in line with the MEDraysintell analysis showing that the growth of nuclear medicine in the future will come through therapeutic radiopharmaceuticals (radiotherapeutics). Revenue for Xofigo – a product used in the treatment of prostate and bone cancers – reached EUR 157 million (US$ 209 million) in 2014.
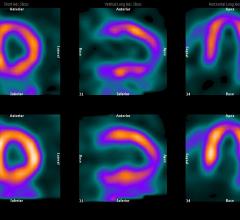
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Researchers at the University of California, San Diego School of Medicine have identified a key piece in the complex molecular puzzle underlying heart failure.
Carroll Hospital Center physicians will now have 24/7 remote access to the University of Maryland Medical Center’s (UMMC) Brain Attack Team through a new telemedicine service for stroke patients.
George Adams, M.D., an interventional cardiologist with North Carolina Heart and Vascular, successfully treated a patient having an acute myocardial infarction (AMI) with the Aspire Mechanical Thrombectomy System.


 March 12, 2015
March 12, 2015