Boston Scientific Corp. announced that its subcutaneous implantable defibrillator (S-ICD System) will have designated Current Procedural Terminology (CPT) Category I codes by the American Medical Association (AMA), effective Jan. 1, 2015.
The American College of Cardiology (ACC), which has been developing clinical practice guidelines in partnership with the American Heart Association (AHA) for more than three decades, has launched a new Guideline Clinical App to serve as the mobile home for all ACC/AHA guideline content and related tools.
Philips Healthcare is introducing its latest mobile c-arm imaging system. The Veradius Unity is uses a thin flat digital detector instead of an image intensifier and offers several features to aid procedural guidance and enhance the user interface.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Covidien releases 12-month results of the DEFINITIVE AR study, the first randomized study designed to identify the clinical benefits of plaque removal using directional atherectomy followed by drug-coated balloon. The results were presented by Professor Thomas Zeller of the Universitaets-Herzzentrum, Bad Krozingen, Germany at the Vascular Interventional Advances (VIVA) 2014 conference in Las Vegas, Nev.
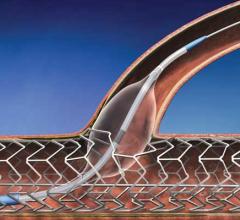
Micell Technologies Inc. announced commercialization plans for its MiStent Sirolimus Eluting Absorbable Polymer Coronary Stent System (MiStent SES). In support of this strategy, the company has entered into a five-year agreement with Stentys as an exclusive worldwide distributor of the MiStent SES, except in the United States, Canada, China, South Korea and Japan. Stentys plans to launch MiStent in the first half of 2015 in Europe, followed by many other geographies where they have built a commercial network
ClearFlow Inc. announced positive results from the Prevention of Retained Blood Outcomes Using Active Clearance Technology trial (PRO-ACT), a clinical study evaluating the use of PleuraFlow Active Clearance Technology System to prevent retained blood complications.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
KLAS releases scores and analysis regarding solutions for electronic medical record (EMR) interoperability and health information exchanges (HIEs). This, to provide clarity into a market that is cluttered with competing claims.
Coquí RadioPharmaceuticals Corp. is working to become the first commercial producer of lifesaving medical isotopes in the United States. In 2012, Congress passed legislation making it a national priority to produce Molybdenum-99, an isotope necessary to detect a wide range of diseases, including cardiovascular disease and cancer.
Optimizing the treatment of coronary artery disease with a new foundation for future stent innovations, Medtronic Inc. announces CE mark and international launch of the Resolute Onyx Drug-Eluting Stent (DES). The first live patient implant of the Resolute Onyx DES occurred recently during the XII International Course of Endovascular and Myocardial Therapy in Madrid, Spain. The Resolute Onyx Drug-Eluting Stent is not approved in the United States.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
St. Mary’s Health Care System installed two Vantage Titan 1.5T MR (magnetic resonance) systems from Toshiba America Medical Systems Inc. St. Mary’s utilizes the systems to enhance its cardiac imaging program and for advanced imaging studies that include myocardial perfusion and stress MR.
The actions — or inaction — of patients should be considered in programs designed to improve care and patient outcomes, according to a report released by the American College of Cardiology, American Heart Association, American Association of Cardiovascular and Pulmonary Rehabilitation, American Academy of Family Physicians and the American Nurses Association in collaboration with other professional organizations.
LifeWatch AG is signing an agreement with Vital Connect Inc. to utilize their HealthPatch MD as a 1-lead ECG device in its cardiac monitoring business. LifeWatch and Vital Connect are working together to integrate the HealthPatch MD into LifeWatch's analytical mobile application and reporting systems with a view to bringing an integrated solution to market in the first half of 2015.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Catalist-listed QT Vascular Ltd. has acquired a novel technology platform called Java, and all associated intellectual property, which was developed independently in Israel.
Five-year results from the largest and longest-running clinical trial of a drug-eluting stent for treating peripheral arterial disease (PAD) confirmed long-term patency for patients treated with Zilver PTX.
TIDI Products LLC has acquired CFI Medical Solutions (CFI) of Fenton, Mich., a diversified medical device manufacturer and engineering resource for hospitals, distributors and global original equipment manufacturers.


 November 14, 2014
November 14, 2014