ReFlow Medical Inc. announced U.S. Food and Drug Administration (FDA) clearance for commercialization of their SpeX shapeable support catheter in the United States for use in the peripheral vasculature, as well as the first American clinical cases with the device. The initial United States cases were performed by John R. Laird, M.D., at the University of California-Davis Vascular Center.
The U.S. Food and Drug Administration (FDA) cleared a new screening test that predicts a patient’s risk of future coronary heart disease (CHD) events, such as heart attacks.
McKesson is committed to working with facilities to adapt to the new healthcare reality in both an effective and cost ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
QICS is a unique, automated workflow management solution to help today's medical professionals enhance the delivery of ...
Having been active in the age of transformation in which manufacturing, retail, finance, high-tech and oil and gas all experienced in the late 1980s and into the 1990s, I realized that healthcare would have its date with enterprise-wide change. Healthcare as an industry has for too long existed in an environment of stasis, now with the rapid pace of technology, competition and exorbitant costs, it is evident to the patient, the competition, the government and the rest of the world that it must adapt to survive. Medical Imaging is a profit center for most healthcare providers and has historically been the hot bed for technology advancement and innovation.
Topera, Inc. announced that its RhythmView 3-D Mapping System received the Most Innovative New Product (MIP) Award at the 2014 CONNECT MIP Awards.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Sapheneia has partnered with Scannerside to provide a comprehensive dose strategy (CDS) to the medical imaging industry.
St. Jude Medical Inc. announced CE Mark approval of the Quadra Allure MP cardiac resynchronization therapy pacemaker (CRT-P). The Quadra Allure MP is a quadripolar CRT-P with the MultiPoint Pacing option, a technology that has been shown to enhance patients’ response to CRT, potentially improving quality of life for patients with heart failure.
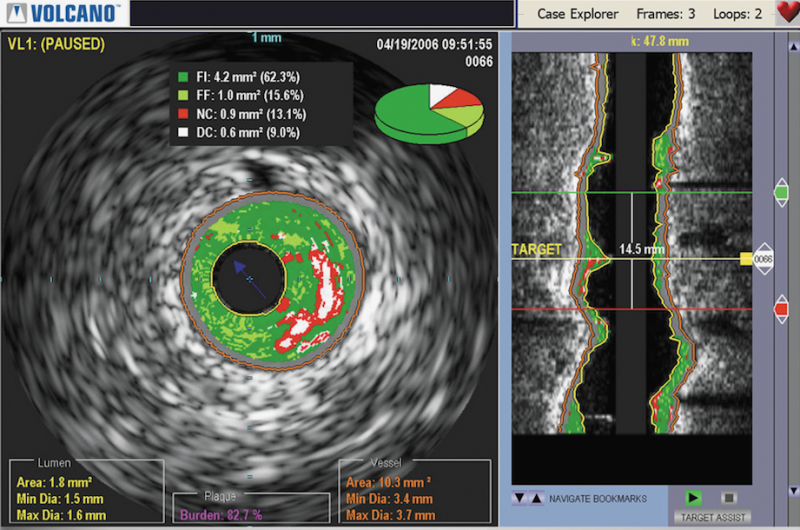
The purchase will enable Philips to further expand its advanced imaging capabilities of its angiography suite. Volcano is a leader in intravascular ultrasound (IVUS) and fractional flow reserve (FFR) catheter measurements used in the interventional cardiology cath lab. The additional technology will enhance Philips' angiography imaging system suite offerings.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

Medtronic Inc. announced the U.S. Food and Drug Administration (FDA) approval and commercial launch of two additional Attain Performa left ventricular (LV) quadripolar leads. Both devices can be paired with the Medtronic Viva Quad XT and Viva Quad S cardiac resynchronization therapy defibrillators (CRT-D) to treat patients with heart failure.
TeraRecon announced the availability of a first-of-kind upgrade program called Any/\One, at RSNA 2014.
Helping connect healthcare experts and increasing the usability of the wealth of medical imaging data is the goal of a cloud-based network solution from Siemens Healthcare.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Alere Inc. has initiated a voluntary correction to inform United States users of the Alere INRatio and INRatio 2 PT/INR Monitor system of certain medical conditions that should not be tested with the system (INRatio Monitor or INRatio2 Monitor and INRatio Test Strips).
Data from a Bayer study in children less than 2 years of age (infants) was presented at the 2014 Radiological Society of North America (RSNA) scientific assembly and annual meeting. The primary endpoint of the study was the evaluation of the pharmacokinetics (PK) of Gadavist (gadobutrol) injection in plasma at the standard dose of 0.1 mmol/kg body weight. Safety was a secondary endpoint and the study also included a qualitative assessment of efficacy.
A faster, coordinated emergency response in collaboration with hospital cardiac catheterization laboratories in each United States region, including New York City, is associated with improving patient survival from a heart attack caused by a sudden, completely blocked artery called an ST-elevated myocardial infarction (STEMI), according to a study presented at the American Heart Association Scientific Sessions 2014.


 December 18, 2014
December 18, 2014











