At EuroPCR 2015, Mitralign presented updates on its mitral and tricuspid valve repair systems, including a presentation on six-month data on its Mitral system.
Edwards Lifesciences Corp. announced that 30-day outcomes for intermediate-risk patients treated transfemorally with the SAPIEN 3 transcatheter aortic valve demonstrated very low mortality and stroke rates, and no severe paravalvular leaks. These independently adjudicated data from centers in Europe and Canada are consistent with the outcomes recently reported in a similar study of 1,000 patients treated at 51 centers in the United States. The study was presented at EuroPCR 2015 by Alec Vahanian, M.D., chair of the cardiology department at Bichat University Hospital, Paris.
St. Jude Medical Inc. announced preliminary results from the ILUMIEN I trial and final results from the ILUMIEN II clinical study. Taken together, the findings from both studies show that with resolution up to 10 times higher than intravascular ultrasound (IVUS), optical coherence tomography (OCT) imaging can help improve stent selection and deployment, better support clinical decision-making and improve patient outcomes.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Orlando Health recently successfully unrolled Lumedx’s physician structured reporting and image management solution across five of its campuses. The implementation was smooth and swift, user adoption is 100 percent, and the benefits are manifold.
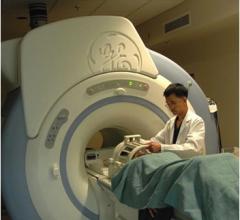
According to a new study, small changes in quality improvement procedures enabled clinicians to use magnetic resonance imaging (MRI) scans to diagnose stroke patients before giving acute treatment, within 60 minutes of arrival. MRI scans provide detailed images but take longer to complete than computed tomography (CT) scans, which are commonly used in most centers.

The cost of bringing innovative new cardiovascular technology to the U.S. market today is staggering, numbering in the tens of millions up to $1 billion. Many in the medical device and drug industries see this as a major barrier to developing new therapies. The majority of this cost is in the preclinical animal testing stage and the human clinical trials required to gain regulatory clearance. New technologies may very soon offer a way to greatly decrease these costs. In addition, these technologies may be able to identify which drugs or device therapies will be effective in humans before spending millions in potentially dead-end research.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
A study in the May issue of the International Journal of Cardiovascular Imaging examines the ability of the EchoInsight software to achieve quantitative assessment of the right ventricle (RV). The study, “Semi-automated Echocardiographic Quantification of Right Ventricular Size and Function," was published by Diego Medvedofsky, Karima Addetia, Roberto Lang, Victor Mor-Avi, et al.
Holographic Optical Technologies has made its Voxgram hologram technology available to the consumer market through its just-launched Kickstarter campaign.
Significantly fewer renal and cardiac events are associated with angioplasty procedures using isosmolar contrast medium (IOCM) agent Visipaque (iodixanol) than those using low-osmolar contrast media (LOCM), according to new data presented at EuroPCR 2015.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Boston Scientific reported positive, long-term data from the EVOLVE Trial of the Synergy everolimus-eluting bioabsorbable polymer platinum chromium coronary stent system, with no new major adverse cardiac events reported between years three and four. The study results were presented at EuroPCR 2015 by Prof. Ian Meredith, director of MonashHeart, at Monash Medical Centre in Melbourne, Australia.
New clinical data from two different studies show the IN.PACT Admiral drug-coated balloon from Medtronic plc successfully treated long lesions in the superficial femoral and popliteal arteries. The data was presented at EuroPCR 2015 during the “Hot line” session on “Peripheral interventions.”
St. Jude Medical Inc. announced new results from two clinical studies further supporting the use of its fractional flow reserve (FFR) technology to optimize percutaneous coronary intervention (PCI) procedures. The studies — a 15-year follow-up to the DEFER study and primary results from the CONTRAST study — were presented during hotline sessions at EuroPCR 2015. Combined, the studies contribute to the growing body of evidence supporting FFR as a valuable and important decision-making tool for physicians.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Direct Flow Medical Inc. announced positive two-year data from the DISCOVER CE Mark Trial studying its Direct Flow Medical Transcatheter Aortic Valve System at the EuroPCR conference in Paris, France. Demonstrating excellent patient outcomes with few complications, the two-year data were presented by Antonio Colombo, M.D., Ph.D., from the Ospedale San Raffaele in Milan, Italy.
PHS Technologies Group LLC, a unit of PACSHealth LLC announced that Dell Healthcare and Life Sciences will become a marketing, distribution and hosting partner for its DoseMonitor OnLine software solution.
The Phase C results of the ProMRI Clinical Study were presented at the late-breaking clinical trial session of the Heart Rhythm Society (HRS) 2015 meeting.

 May 26, 2015
May 26, 2015