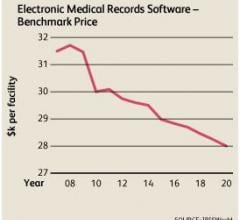
According to market research firm IBISWorld, the average price of electronic medical record (EMR) software is forecast to continue declining at an average annual rate of 0.7 percent through 2019.
Jersey Shore University Medical Center, part of Meridian CardioVascular Network, is the first hospital in New Jersey to implant the Micra Transcatheter Pacing System (TPS), which Medtronic calls the world’s smallest pacemaker.
Amaranth Medical announced that it completed enrollment in May in the RENASCENT-II study of its novel Aptitude 120-micron sirolimus-eluting bioresorbable scaffold (BRS).
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Biotronik announced results establishing non-inferiority of the Orsiro hybrid drug-eluting stent (DES) to the Resolute Integrity DES were presented during a Hotline Session at EuroPCR 2016.
June 3, 2016 — Cardionovum GmbH recently announced the completion of enrollment of the RAPID trial. Results will be used ...
According to data from a new survey, nearly all of the nation’s hospitals have adopted certified electronic health records (EHRs). The survey was released at the Office of the National Coordinator for Health Information Technology’s (ONC) 2016 annual meeting, May 31-June 2 in Washington, D.C.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
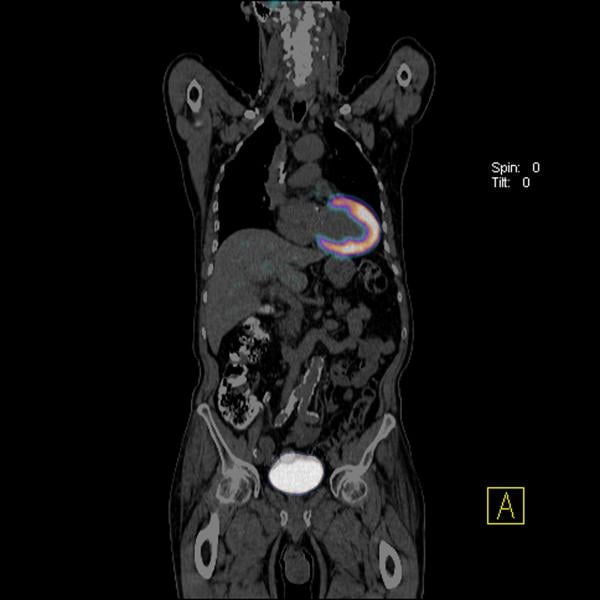
Positron emission tomography (PET) is a nuclear imaging technology (also referred to as molecular imaging) that enables ...
Female atrial fibrillation patients are less likely than their male counterparts to receive blood thinning therapies to prevent stroke, say University of Cincinnati College of Medicine researchers.
June 2, 2016 — Artec 3D, a developer and manufacturer of professional 3-D hardware and software, announced the release ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
June 2, 2016 — Here are the top 20 most popular current articles, news releases and videos on the Diagnostic and ...

Healthcare reform efforts of recent years have challenged the industry to reduce the costs of healthcare for both ...
The American Society of Echocardiography (ASE) will host its 27th Annual Scientific Sessions, June 10-14, 2016, at the Washington State Convention Center in Seattle.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
BioVentrix Inc. announced that it has received a U.S. Food and Drug Administration (FDA) Investigational Device Exemption (IDE) approval to initiate its pivotal clinical trial, named ALIVE (American Less Invasive Ventricular Enhancement).
Biotronik announced the launch of CardioMessenger Smart in the United States. CardioMessenger Smart is a portable monitoring device, about the size of a modern smartphone, that keeps pacemaker, implantable cardioverter defibrillator (ICD) and insertable cardiac monitor (ICM) patients connected to their physician remotely, enabling more efficient care management anywhere in the world.
A person is admitted to the hospital with a stroke, but not much is known about whether or not that patient will undergo neuroimaging.

 June 07, 2016
June 07, 2016