In the past few years, concern has skyrocketed from interventional cardiologists and cath lab staff over radiation dose ...
August 9, 2016 — The U.S. Food and Drug Administration (FDA) granted market approval for the Emblem MRI Subcutaneous ...
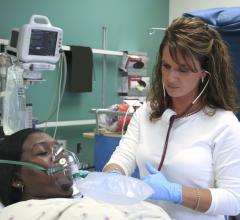
August 9, 2016 – With the use of intensive care units (ICUs) on the rise in many hospitals, researchers at LA BioMed and ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Updated recommendations from the American Academy of Neurology (AAN) states that catheter-based closure should not be routinely recommended for people who have had a stroke and also have a heart defect called a patent foramen ovale (PFO), a channel between the top two chambers in the heart.
ClearFlow Inc. announced in late July that its PleuraFlow Active Clearance Technology is now available for the treatment of pediatric cardiothoracic surgery patients.
August 5, 2016 — The Centers for Medicare and Medicaid Services (CMS) has reassigned MitraClip transcatheter mitral ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
August 5, 2016 — Osprey Medical Inc. said it received commitments from investors for approximately $28 million ...

August 5, 2016 — Here are the top 20 most popular current content on the Diagnostic and Interventional Cardiology (DAIC) ...

August 4, 2016 — Medtronic announced CE (Conformité Européenne) mark market clearnace for the self-expanding ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
As more procedures move into the cath lab, growing complexity is fueling an increase in procedural volume and the need ...
August 3, 2016 — The U.S. Food and Drug Administration (FDA) has granted market clearance to Rex Medical’s bioresorbable ...

The Society of Cardiovascular Computed Tomography (SCCT) annual meeting offers an in-depth review of all aspects of ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
August 2, 2016 —The U.S. Food and Drug Administration (FDA) granted market clearance for W. L. Gore & Associates’ Gore ...
According to a new study, the last 13 years have seen substantial growth in the ordering of computed tomography angiography (CTA) examinations in the Medicare population, particularly in the emergency department (ED) setting.

Patients with atrial fibrillation (AF or Afib) are high risk for stroke due to the formation of thrombus emboli in the ...

 August 09, 2016
August 09, 2016