BioTelemetry Inc. announced in late August that the company has received CE mark approval of its Holter analysis software. The software can be used either on a stand-alone basis or in combination with the company’s family of digital Holter recorders.
Nearly 2 out of 5 people with diabetes who could benefit from statin therapy to lower their risk of future heart attack, stroke and related death were not prescribed one, according to a research letter published in the Journal of the American College of Cardiology. The analysis also showed wide variation in statin use across cardiology practices included in the study.
C. R. Bard Inc. announced the U.S. Food and Drug Administration (FDA) approved an Investigational Device Exemption (IDE) supplemental application to modify the primary endpoint to a six-month time point for the Lutonix 014 Drug Coated Balloon PTA Catheter (DCB). The Lutonix 014 device is currently the only DCB in an IDE clinical trial in the United States for treatment of arteries below the knee (BTK).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Smartphone communication among medical teams at different hospitals can significantly reduce the time it takes for heart attack patients to get lifesaving treatment after a hospital transfer, according to a research letter published in the Journal of the American College of Cardiology.
A Google search for heart conditions will now prominently display important questions patients should ask their doctor based on clinical guidelines developed by the American College of Cardiology.
Technology advances coupled with increased use of social media and personal devices could offer new possibilities for treating patients and improving outcomes, but new approaches must be rigorously evaluated, according to U.S. Food and Drug Administration Commissioner Robert M. Califf, M.D., MACC. Califf’s comments appeared in a column published in the Journal of the American College of Cardiology.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
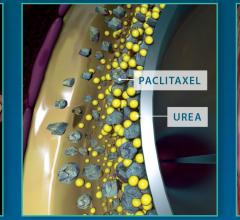
New data presented at the Vascular Interventional Advances (VIVA) conference demonstrated the durability, consistency and safety of Medtronic’s IN.PACT Admiral drug-coated balloon. The presentation included three-year results from the pivotal IN.PACT SFA Trial and one-year, real-world results from the full clinical cohort of the IN.PACT Global Study.
4Tech Inc. announced that its TriCinch device has been used in the world’s first-ever successful transcatheter tricuspid valve repair without the use of transesophageal echocardiography (TEE) or general anesthesia to successfully treat tricuspid regurgitation (TR). The TriCinch implant took less than one hour and allowed substantial reduction of TR.
September 22, 2016 — GE Healthcare announced the global commercial launch of its new generation of high-end portable ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

The rapid growth of transcatheter structural heart procedures and the need for increased use of echocardiography as an ...
With bundled payments putting increased pressure on hospitals to manage supply costs while providing quality patient ...
Claret Medical announced its filing of a marketing application with the U.S. Food and Drug Administration (FDA) for clearance of the Sentinel Cerebral Protection System (CPS). The Sentinel CPS is designed to protect the brain by capturing and removing debris dislodged during transcatheter aortic valve replacement (TAVR) that enters cerebral circulation and carries the potential for stroke. There are currently no cerebral embolic protection technologies available in the United States to protect TAVR patients from cerebral embolic events, according to Claret Medical.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Medtronic plc announced the results of an analysis that reveals patients increasingly adhere to heart failure medications after they receive cardiac resynchronization therapy (CRT) devices. The analysis of more than 4,500 patients revealed that compliance with their medications increased 67 percent 24 months after receiving their CRT implants, compared to the 24 months prior to implant (p<0.001). The results were presented at the 2016 Heart Failure Society of America (HFSA) Scientific Meeting in Orlando, Fla.
September 20, 2016 — Teleflex Inc. recently displayed its vascular access technologies and a new educational platform ...
September 19, 2016 — Edwards Lifesciences received European CE mark to expand use of the Edwards Sapien 3 transcatheter ...


 September 27, 2016
September 27, 2016