The U.S. Food and Drug Administration (FDA) has issued 510(K) Class II clearance to a web client for the AirStrip One mobile interoperability platform and application. The system can be run on desktops and laptops using Internet Explorer and Google Chrome.
October 10, 2016 — ZipLine Medical Inc. recently announced results from a study recently published online in Pacing and ...
Francesco Maisano, clinic director at the University Hospital Zurich, recently led a team of cardiac surgeons and cardiologists in for the first time repairing a leaky tricuspid valve using a new catheter technology.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
St. Jude Medical Inc. announced the U.S. clearance and launch of the company’s new PressureWire X Guidewire fractional flow reserve (FFR) measurement system. The latest generation of the PressureWire Guidewire system is designed to offer improved shapeability and better shape retention aimed at reducing vessel trauma, with the accuracy and simplicity physicians expect when treating patients during percutaneous coronary intervention (PCI), especially those with complex anatomies.

The transradial revolution is one of the fastest growing trends in cardiology. Compared to the femoral access technique ...
Mercator MedSystems recently announced that 13-month data from the DANCE trial was presented during a late-breaking scientific session at the Vascular Interventional Advances (VIVA) Annual Conference 2016. DANCE is a prospective, multicenter, single-arm study designed to assess the clinical performance of the localized delivery of a generic steroid, dexamethasone, to the tissues around arteries that have been injured during endovascular interventions, using Mercator’s proprietary Bullfrog Micro-Infusion Device.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
October 7, 2016 — A Northwestern Medicine cardiac surgeon was recently the first in Illinois and second in the United ...
October 6, 2016 — Xeltis announced this week that patients implanted with its bioabsorbable cardiovascular conduit ...
St. Jude Medical Inc. announced a full market release of its EnSite Precision cardiac mapping system and new Sensor Enabled tools in Europe. The new platform is now installed and active in more than 100 sites across Europe and has been used to support more than 5,000 ablation cases since the system’s CE Mark approval in January 2016.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Interventional labs now have an imaging system that provides clinicians flexibility to perform a wide array of procedures with the launch of Toshiba’s Infinix-i Sky +*. The ceiling-mounted system features a double sliding C-arm and 12 x 16-inch flat panel, offering clinicians the potential to increase coverage, speed and patient access.
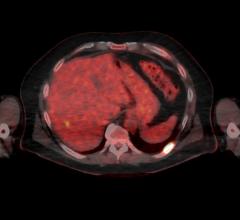
GE Healthcare recently announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its Discovery MI digital positron emission tomography (PET)/computed tomography (CT) system and shared a series of first clinical images. Built with technology allowing significantly better small lesion detectability, Discovery MI can help clinicians in their efforts to diagnose and stage disease earlier.
October 5, 2016 — Vascular Solutions Inc. initiated a nationwide recall of its Twin-Pass Dual Access catheters used in ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
October 5, 2016 — Medtronic said two previously communicated global voluntary recalls related to the HeartWare ...
GE Healthcare announced that the U.S. Food and Drug Administration (FDA) has approved a label change for the ultrasound contrast agent Optison (Perflutren protein-Type A Microspheres Injectable Suspension, USP). The FDA removed the contraindications for use in patients with cardiac shunts and for administration by intra-arterial injection. Both contraindications have been revised and moved to the WARNINGS AND PRECAUTIONS section (5.3 : Systemic Embolization) of the Full Prescribing Information.
October 4, 2016 — Xeltis recently announced that three pediatric patients have been successfully implanted with the ...

 October 11, 2016
October 11, 2016