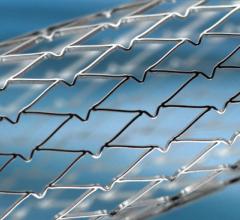
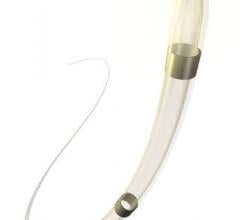
Medtronic plc last week unveiled new health economic analysis data from the FIRE AND ICE trial that favor cryoballoon catheter ablation over radiofrequency (RF) ablation related to trial period cost savings. The savings came as a result of fewer cardiovascular (CV) rehospitalizations and repeat ablations.
The Centers for Disease Control and Prevention (CDC) is warning healthcare providers and patients about the potential risk of infection from certain devices used during open-heart (open-chest) surgery.
Philips announced at The American College of Emergency Physicians' (ACEP) annual meeting that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its S4-1 cardiac transducer for Lumify, its smart-device diagnostic ultrasound solution. The pocket-sized and lightweight S4-1 transducer now offers advanced sensitivity and high-resolution 2-D image quality, along with new exam pre-sets, allowing clinicians to quickly triage and assess their patients.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
As hospitals begin replacing their first-generation 64-slice computed tomography (CT) scanners after a decade of use ...

The Department of Health & Human Services (HHS) today finalized a landmark new payment system for Medicare clinicians that will continue the administration’s progress in reforming how the healthcare system pays for care. The Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) Quality Payment Program, which replaces the flawed Sustainable Growth Rate (SGR), will equip clinicians with the tools and flexibility to provide high-quality, patient-centered care.
Biotricity Inc. announced recently that it will be expanding its existing research partnership with the University of Calgary. In order to develop and validate the next generation of medically relevant wearable monitors, the focus of the new partnership will be on areas beyond cardiac medicine, including fetal monitoring and sleep apnea.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Medtronic plc is the first company to receive U.S. Food and Drug Administration (FDA) approval for its suite of cardiac rhythm and heart failure devices and leads to be scanned in both 3 and 1.5 Tesla (T) magnetic resonance imaging (MRI) machines. This advancement gives patients with Medtronic SureScan MR-conditional pacemakers, implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy-defibrillators (CRT-Ds) and leads access to MRI scans on any part of the body.
A surveillance project to evaluate the safety and effectiveness of transcarotid artery revascularization (TCAR) in comparison with carotid endarterectomy (CEA) is being launched by the Society for Vascular Surgery Patient Safety Organization (SVS PSO). Carotid artery stenting (CAS) and CEA are performed in patients with atherosclerotic narrowing of the carotid artery in order to reduce stroke risk.
SentreHeart Inc. announced this week it has treated the first patients using the Eclipse Surgical Device, which is approved and CE Marked in Europe for left atrial appendage (LAA) closure. Krzysztof Bartus, M.D., Ph.D., and associate professor of the Jagiellonian University, Department of Cardiovascular and Transplant Surgery in Krakow, Poland performed the procedures.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
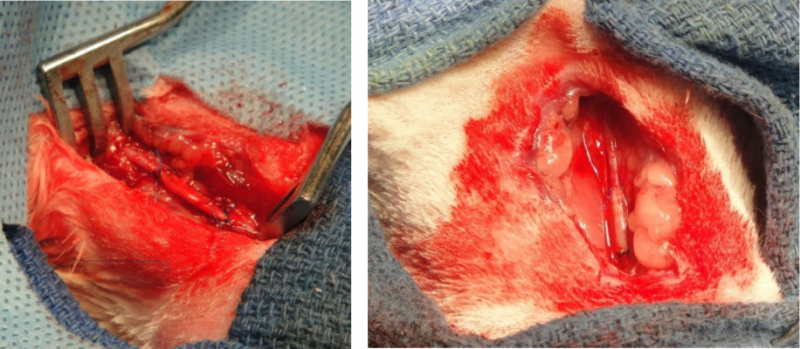
The U.S. Food and Drug Administration (FDA) changed its rules concerning custom medical devices Oct. 12, adding new ...
VIVA (Vascular Interventional Advances) Physicians announced a number of highly anticipated late-breaking clinical trial results at VIVA 16, hosted at the Wynn Las Vegas Sept. 18-22.
October 12, 2016 — The Society for Vascular Surgery (SVS) has released new reporting standards focused on endovascular ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...

While guidewires are a key tool used by all interventionalists in the cath lab, most operators do not have a deep ...
Medtronic plc announced recently that the U.S. Food and Drug Administration (FDA) has cleared the TrailBlazer angled support catheter for use in the peripheral vascular system. Support catheters such as the Trailblazer are often used in endovascular procedures treating complex peripheral artery disease (PAD).
Awards from the National Institutes of Health’s Common Fund are supporting research on the peripheral nervous system, in hopes of finding new ways to treat conditions such as asthma, diabetes and nausea. The new awards total more than $20 million in fiscal year 2016, and go to 27 multidisciplinary research teams through the Stimulating Peripheral Activity to Relieve Conditions (SPARC) program. The SPARC program plans to support awards totaling approximately $238M through fiscal year 2021, pending available funds.


 October 17, 2016
October 17, 2016