Svelte Medical Systems Inc. announced this week it received CE Mark certification of the Direct Sirolimus-Eluting Coronary Stent Rapid-Exchange (RX) System for the treatment of coronary artery disease. The first patient to receive Direct RX was treated by Auke Weevers, M.D., a practicing interventional cardiologist at Albert Schweitzer Ziekenhuis in Dordrecht, The Netherlands.
This video, provided by Zoll, demonstrates how cardiologists can explain sudden cardiac death to patients. It is ...
October 28, 2016 – Tryton Medical Inc., a primary developer of stents to treat coronary bifurcation lesions, and ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
With quality of care and cost efficiency at the top of your mind, there is no room in your hospital for waste from high ...
October 28, 2016 — Medicure Inc. recently received U.S. Food and Drug Administration (FDA) market clearance for its new ...
Acutus Medical announced the completion of the first patient procedure in the “Utilizing Novel Dipole Density Capabilities to Objectively Visualize the Etiology of Rhythms in Atrial Fibrillation” (UNCOVER-AF) clinical study. The study will evaluate the incidence of device and procedure-related safety, effectiveness and efficiency using the AcQMap High Resolution Imaging and Mapping System to guide ablation in persistent atrial fibrillation (AF) patients.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Volume matters when it comes to who does certain procedures to removing heart-attack causing blockages from arteries, according to a new study published recently in JACC: Cardiovascular Interventions.
October 27, 2016 — Corindus Vascular Robotics Inc. announced it received 510(k) clearance from the U.S. Food and Drug ...

The volume of information attendees receive at the annual Transcatheter Cardiovascular Therapeutics (TCT) interventional ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
October 27, 2016 — BioTrace Medical Inc. said it received U.S. Food and Drug Administration (FDA) 510(k) clearance for ...
Philips recently announced its latest image guidance solutions to be featured at the 2016 Transcatheter Cardiovascular Therapeutics meeting (TCT) in Washington, D.C., Oct. 29 – Nov. 2, 2016. Highlights will include the introduction of instant wave-Free Ratio (iFR) co-registration, which integrates iFR pullback data with the angiogram, and the third generation of HeartNavigator, live image guidance software for advanced structural heart disease procedures.
October 26, 2016 — The U.S. Food and Drug Administration (FDA) provided an update and additional information regarding ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
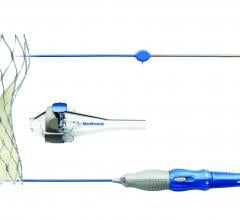
Medtronic announced the U.S. Food and Drug Administration (FDA) approval and U.S. launch of the CoreValve Evolut R 34 mm valve, now the largest sized transcatheter aortic valve replacement (TAVR) system available in the United States.
Stereotaxis Inc. and Robert Wood Johnson University Hospital (RWJUH) announced that Zyad Younan, M.D., has completed more than 500 cardiac ablation procedures using the Niobe remote magnetic navigation system. Younan currently leads the Northeast region in catheter ablations performed using the Niobe system in the six-and-a-half years since installation.
A recent study from University of Alabama at Birmingham (UAB) researchers published in PLOS ONE compares different available treatments for stroke prevention in patients with non-valvular atrial fibrillation (NVAF).

 October 28, 2016
October 28, 2016