May 15, 2017 – The Heart Rhythm Society (HRS) in joint partnership with several other electrophysiology societies issued ...
A first-of-its-kind study discovered that women and minorities who underwent a percutaneous coronary intervention (PCI) are at greater risk of experiencing recurrent cardiac events within the first year after their procedure compared to Caucasian men. Those outcomes may be attributable to their race, gender and socioeconomic status rather than the PCI procedure itself. Results from “Interaction Effects of Race/Ethnicity and Sex on Outcomes after PCI: A Subanalysis of the PLATINUM Diversity study” were presented as a late-breaking clinical trial at the Society for Cardiovascular Angiography and Interventions (SCAI) 2017 Scientific Sessions May 10-13 in New Orleans.
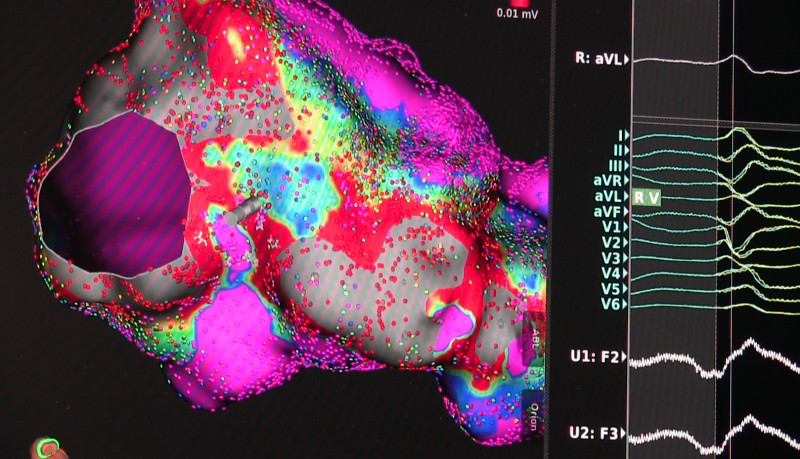
May 15, 2017 — The Heart Rhythm Society (HRS) released a first-of-its-kind consensus statement in the United States on ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
For patients who undergo transcatheter aortic valve replacement (TAVR), their risk factors, not the type of valve used, determined their 30-day post-TAVR outcomes. Results from “Impact of valve design and bivalirudin vs. unfractionated heparin for anticoagulation in transcatheter aortic valve replacement: Results from the BRAVO-3 trial” were presented as a late-breaking clinical trial at the Society for Cardiovascular Angiography and Interventions (SCAI) 2017 Scientific Sessions, May 10-13 in New Orleans.
In a large-scale analysis of percutaneous coronary intervention (PCI)-related hospitalizations, people admitted to the hospital on a weekend were twice more likely to die than those hospitalized on a weekday. Results from “Weekend Effect for Percutaneous Coronary Intervention Admissions: A 10-Year U.S. Experience” were presented at the Society for Cardiovascular Angiography and Interventions (SCAI) 2017 Scientific Sessions, May 10-13 in New Orleans.
May 15, 2017 – The U.S. Food and Drug Administration (FDA) has granted market clearance Medtronic’s portfolio of ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
A study in 65 countries has revealed low adoption of International Atomic Energy Agency (IAEA) recommendations to reduce nuclear cardiology radiation exposure. The research was presented at ICNC 2017, May 7-9 in Vienna Austria, by Edward Hulten, M.D., a cardiologist at the Walter Reed National Military Medical Center, Bethesda, Md.
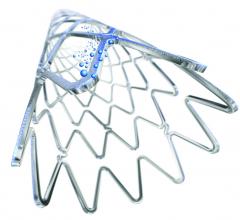
PinnacleHealth CardioVascular Institute enrolled the first patient in Pennsylvania in a trial assessing the safety and effectiveness of a new stent to treat patients with coronary heart disease who are at higher risk for bleeding.
An analysis of data from the entire development program consisting of three trials assessing the feasibility of using a stem cell therapy (CD34+ cells) to treat patients with refractory angina showed a statistically significant improvement in exercise time as well as a reduction in mortality. Results from “CD34+ Stem Cell Therapy Improves Exercise Time and Mortality in Refractory Angina: A Patient Level Meta-Analysis” were presented as a late-breaking clinical trial at the Society for Cardiovascular Angiography and Interventions (SCAI) 2017 Scientific Sessions, May 10-13 in New Orleans.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 12, 2017 – New study proves safety of novel, wireless pacing system, WiSE CRT (Wireless Stimulation Endocardially ...

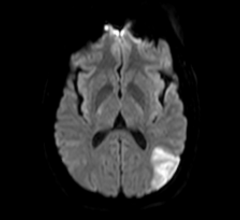
May 12, 2017 – A new study found that dementia rates increase with delays in starting anticoagulation treatment for ...
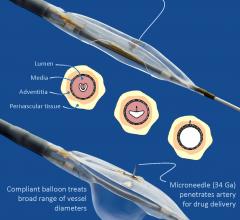
Peripheral artery disease (PAD) patients who were treated with an anti-inflammatory steroid injected directly into the tissue surrounding their leg artery showed a significant reduction in inflammatory biomarkers, according to new data from the DANCE trial. Results from the trial were presented as a late-breaking clinical trial at the Society for Cardiovascular Angiography and Interventions (SCAI) 2017 Scientific Sessions, May 10-13 in New Orleans.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
More than one-third of patients undergoing transcatheter aortic valve replacement (TAVR) were observed to have atrial fibrillation (AF) either at baseline or new-onset within 30 days after TAVR, according to data from the BRAVO-3 randomized trial. Patients with new-onset AF had a greater than 4-fold greater risk of stroke within 30 days. Results from the trial were presented as a late-breaking clinical trial at the Society for Cardiovascular Angiography and Interventions (SCAI) 2017 Scientific Sessions, May 10-13 in New Orleans.
May 11, 2017 — Biotronik announced U.S. Food and Drug Administration (FDA) approval of the company's MultiPole Pacing ...
May 11, 2017 — A new study shows that the Apple Watch's heart rate sensor, when paired with an artificial intelligence ...

 May 15, 2017
May 15, 2017