ECRI Institute announced a new mergers and acquisitions service that empowers new hospital alliances to control their spending and plan for the future by eliminating waste and redundancy in medical-surgical supplies and capital medical equipment.
Warfarin is a commonly prescribed blood thinner used to prevent harmful blood clots. However, the drug requires frequent monitoring, daily dosing and can result in serious negative effects when mixed with vitamin K, a vitamin commonly found in vegetables such as lettuce or broccoli. Now, a new study from University of Missouri Health Care has found that using electronic health records (EHR) can improve the care patients receive after they leave the hospital and eliminate potential confusion among care providers and pharmacists.
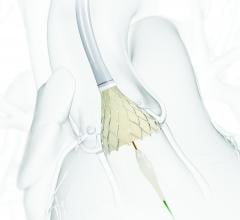
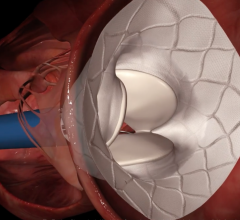
Medtronic plc announced the CE (Conformité Européenne) mark and European launch of the CoreValve Evolut R 34 mm valve — the largest sized transcatheter aortic valve implantation (TAVI) system available in Europe. The new Evolut R 34 mm valve is approved for severe aortic stenosis patients who are at intermediate, high or extreme risk for surgery with an annulus size ranging from 26-30 mm. This large valve segment is estimated to account for approximately 20-25 percent of the eligible European TAVI patient population. Previously, some of these patients were unable to receive a TAVI due to the larger size of their native diseased aortic valve.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Of patients over age 65 who received an implantable cardioverter-defibrillator (ICD) after surviving sudden cardiac arrest or a near-fatal arrhythmia, almost 80 percent survived two years, according to a new study in the Journal of the American College of Cardiology. This is a higher rate than found in past trials performed to demonstrate the efficacy of the devices in this situation.
Lumedx Corp. recently announced that it has implemented Phase 1 of a long-term, multiphase cardiovascular information system (CVIS) project with Baylor Scott & White Health.
Investigators at the University of Utah have identified distinct differences in the hearts of advanced heart failure patients who have defied the odds and showed signs of recovery from the disease. Published online in the journal Circulation, the new findings could help clinicians identify the best candidates for cardiac recovery therapies.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The new SunTech Oscar 2 24-hour ambulatory blood pressure monitoring system is now available with the True24 ABPM Patient Diary mobile app, which links to the Oscar 2 via Bluetooth. The True24 app provides a tutorial on ABPM, and prompts the patient to enter information about their activity, posture or any symptoms experienced during a physician-prescribed ABPM study. This diary information helps clinicians interpret the ambulatory blood pressure study data to make well-informed hypertension treatment decisions.
James Laskaris, emerging technology analyst at MD Buyline, recently offered his perspective on new medical technology innovations that might be just months or a few years away.

One of the big advancements in drug-eluting stent (DES) technology has been the development of bioresorbable polymers ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Frost & Sullivan has released a new report, “Vision 2025 – Future of Healthcare,” part of the company’s Advanced Medical Technologies, that identifies 18 technologies that will impact healthcare paradigms by 2025.
January 16, 2017 — BioSig Technologies Inc. announced that the company’s Pure EP System, a novel cardiac ...
Each year imaging system manufacturers use the Radiological Society of North America (RSNA) meeting at the end of the ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
January 16, 2017 – In 2015, researchers and physicians at the Minneapolis Heart Institute Foundation (MHIF) were the ...
BioTrace Medical Inc. announced in December the first commercial use of the company’s Tempo Temporary Pacing Lead since U.S. Food and Drug Administration (FDA) 510(k) clearance in October 2016.
January 13, 2017 — Results from TRANSFORM-OCT, a prospective, randomized trial using optical coherence tomography (OCT) ...

 January 17, 2017
January 17, 2017