
Medical image analysis based on artificial intelligence (AI) employ convolutional neural networks, support vector ...
July 17, 2017 — Despite issues with point-of-care INR testing company Alere Inc., Abbott announced this week it is once ...
Intact Vascular Inc. announced the U.S. Food and Drug Administration (FDA) approved an Investigational Device Exemption (IDE) supplemental application to modify the primary endpoint in the Tack Optimized Balloon Angioplasty II Below the Knee (TOBA II BTK) clinical trial from 12 months to 6 months.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
A review appearing in the July 18 issue of the Journal of the American College of Cardiology (JACC) discusses current and next-generation implantable hemodynamic monitors. The review particularly examines new approaches focused on the direct measurement of left atrial pressure (LAP), seeking to expand the use of pressure-guided congestive heart failure (CHF) management.
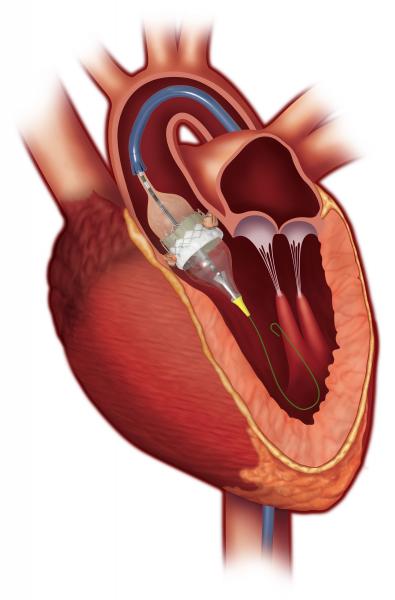
July 14, 2017 — Morristown Medical Center, part of Atlantic Health System, has randomized the first patient in the world ...
In a series of proposed rule changes to the Medicare Physician Fee Schedule (MPFS) released July 13, the Centers for Medicare and Medicaid Services (CMS) pushed back the start date for when providers will be required to consult clinical decision support (CDS) for advanced diagnostic imaging to 2019. CMS indicated the first year would be considered an “educational and operational testing year,” meaning claims would still be paid on advanced diagnostic imaging services that do not indicate appropriate use criteria (AUC) were consulted.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
As the debate over healthcare insurance coverage continues, Senate Republicans on Thursday unveiled the revised version of the Better Care Reconciliation Act (BCRA) to repeal and replace Obamacare after the first attempt was met with strong opposition within their own party. The changes in the revised version are aimed at appeasing more moderate Republicans and expanding aid for lower-income people.
Cardiologs Technologies SAS announced that it has received U.S. Food and Drug Administration (FDA) clearance of its Cardiologs ECG Analysis Platform, a cloud-based cardiac monitoring-analysis web service powered by artificial intelligence (AI). Cardiologs aids physicians in screening for atrial fibrillation (AFib) and other arrhythmias using long-term ambulatory electrocardiogram (ECG) monitoring recordings. The Cardiologs system is also CE marked in Europe.
July 13, 2017 — Experts in heart failure management gathered in June to discuss varying scientific evidence in their ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
July 13, 2017 — About 5.7 million adults in the U.S. suffer from heart failure, and because of a dangerous buildup of ...
William Abraham, M.D., director of the Division of Cardiovascular Medicine at The Ohio State University Wexner Medical ...
Randall Thompson, M.D., outlines three new CPT codes for FFR-CT, a smart phone-based single-lead ECG system and PET ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
June 12, 2017 — Researchers have evaluated the long-term efficacy and safety of long-duration dual anti-platelet therapy ...
July 12, 2017 — The American Medical Association (AMA) has granted a Category III Tracking Code for estimated coronary ...
July 12, 2017 — At the 2017 annual meeting for the Society of Cardiovascular Computed Tomography (SCCT), Ziosoft showed ...

 July 17, 2017
July 17, 2017
















