The double kissing (DK) crush two-stent technique was associated with a lower rate of target lesion failure at one year compared with provisional stenting (PS) in the treatment of true distal bifurcation lesions of the left main artery, according to a large-scale randomized trial.
Analytics 4 Life announced it will be presenting new clinical data on the company's ongoing Coronary Artery Disease Learning and Algorithm Development (CADLAD) study at the Transcatheter Cardiovascular Therapeutics (TCT) 2017 scientific symposium, Oct. 29-Nov. 2 in Denver.
James A. Brink, M.D., FACR, chair of the American College of Radiology (ACR) Board of Chancellors, met with the House Committee on Science, Space and Technology Subcommittee on Energy to urge more research on low-dose medical radiation effects to inform future safety practices. He also told the Committee that medical imaging and radiation oncology save lives.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
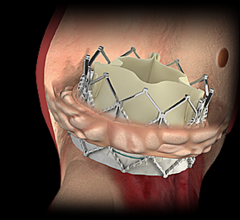
Analysis of the PARTNER 2A trial and the SAPIEN-3 Intermediate Risk registry found transcatheter aortic valve replacement (TAVR) to be highly cost-effective compared with surgical aortic valve replacement (SAVR) in intermediate surgical risk patients with aortic stenosis.
November 1, 2017 — Results from the prospective, randomized, multicenter CULPRIT-SHOCK trial found an initial strategy ...
October 31, 2017 — The VAMPIRE 3 study evaluated if the selective use of distal filter protection might decrease the ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Edwards Lifesciences Corp. announced new data demonstrating substantial economic advantages of the Edwards Sapien 3 transcatheter aortic heart valve for patients suffering from severe, symptomatic aortic stenosis (AS) who are at intermediate risk for open-heart surgery. Results of the economic analysis, which is the first-of-its-kind report on intermediate-risk patients, were presented as a late-breaking clinical trial at the 29th Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation, Oct. 29-Nov. 2 in Denver.
Thirty-day results from ABSORB IV, the largest randomized everolimus-eluting bioresorbable vascular scaffold (BVS) trial to date, found BVS to be noninferior to a cobalt-chromium everolimus-eluting stent (CoCr-EES) for target lesion failure (TLF).
October 31, 2017 — The U.S. Food and Drug Administration (FDA) issued a warning to healthcare providers that interim ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
October 30, 2017 — New study results from the EXCEL trial comparing the quality of life (QoL) of patients with left main ...
The American College of Cardiology, along with the American Heart Association and the Heart Rhythm Society, published new guidelines for the treatment of patients with ventricular arrhythmias and the prevention of sudden cardiac death.
October 30, 2017 — Researchers have discovered a previously unrecognized healing capacity of the heart. In a mouse model ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
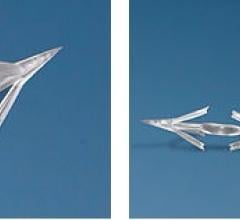
Micro Interventional Devices Inc. (MID) recently announced the second successful implantation of its MIA (Minimally Invasive Annuloplasty) technology. The patient was enrolled in the first arm of the STTAR (Study of Transcatheter Tricuspid Annular Repair) clinical trial studying the safety and efficacy of MID's MIA device designed to eliminate or greatly reduce tricuspid regurgitation.
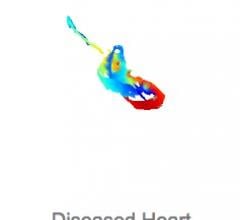
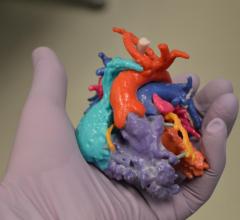
Entering their pre-market phase of development, Materialise is working with select U.S. and EU hospital partners to validate the importance of accurate 3-D modeling to help physicians plan complex transcatheter mitral valve replacement and repair (TMVR/r) procedures.
CeloNova BioSciences Inc. announced the U.S. Food and Drug Administration (FDA) approved expansion of CeloNova's ongoing clinical trial of its proprietary Cobra PzF NanoCoated Coronary Stent (NCS) with 14-day dual antiplatelet therapy (DAPT) in complex patients, such as those who are at high bleeding risk. The COBRA REDUCE trial is the world's first and only randomized control trial to assess 14-day DAPT after percutaneous coronary intervention (PCI), according to the company.


 November 01, 2017
November 01, 2017