Claret Medical announced that since U.S. Food and Drug Administration (FDA) clearance in June 2017, its Sentinel Cerebral Protection System has been adopted by 50 U.S. centers as part of its controlled rollout. The company said that some institutions have adopted the device as an emerging standard of care in the U.S. to protect patients from the risk of stroke by capturing and removing debris associated with transcatheter aortic valve replacement (TAVR) before it travels to the brain. The novel system has been shown to significantly reduce the risk of stroke in the first three days after TAVR by more than 60 percent, according to Claret Medical.

For any cardiology department planning to upgrade its cardiovascular picture archiving and communication system (cardiac ...
February 16, 2018 — California’s Petaluma Health Center (PHC) was recently awarded a 2017 Healthcare Information and ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The father of transradial artery access, Ferdinand Kiemeneij, M.D., Ph.D., interventional cardiologist, The Netherlands ...
DAIC Editor Dave Fornell previews the launch of augmented reality (AR) technology in the March/April 2018 issue of DAIC ...
There are few downsides to using tibial venous access to treat deep vein thrombosis (DVT) with catheter-directed ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The U.S. Food and Drug Administration (FDA) has expanded the indication for Stryker's Trevo Retriever as a front-line treatment for patients experiencing acute ischemic stroke up to 24 hours from symptom onset, increasing the treatment window by 18 hours. The expanded indication of Stryker's clot-removal device is in line with the recently updated treatment guidelines from the American Heart Association (AHA) and American Stroke Association (ASA), and has the potential to reduce disability and improve quality of life for tens of thousands of additional stroke patients each year.
Abiomed Inc. announced that it received an expanded U.S. Food and Drug Administration (FDA) Pre-Market Approval (PMA) for its Impella 2.5, Impella CP, Impella 5.0 and Impella LD heart pumps to provide treatment for heart failure associated with cardiomyopathy leading to cardiogenic shock. This approval expands the previous FDA indication for acute myocardial infarction (AMI) cardiogenic shock and post-cardiotomy cardiogenic shock (PCCS), received in April 2016.
February 15, 2018 — The National Institutes of Health (NIH) awarded a $2.2 million research grant to healthcare ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
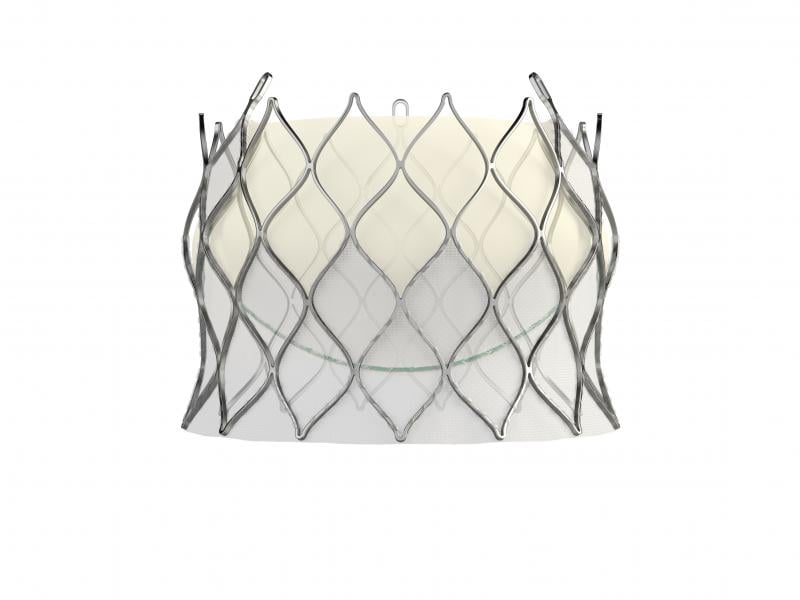
February 15, 2018 — Edwards Lifesciences Corp. has received European CE mark clearance for its self-expanding Centera ...
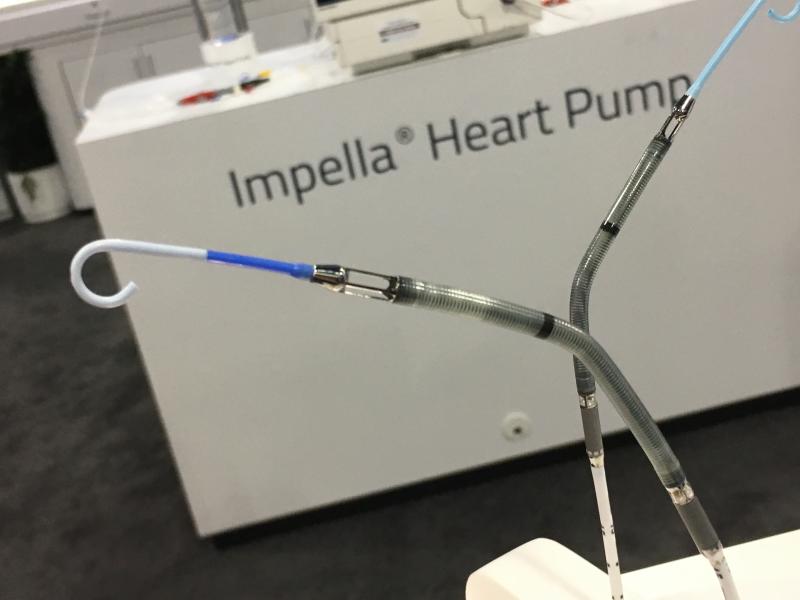
February 14, 2018 — Abiomed Inc. announced it received an expanded U.S. Food and Drug Administration (FDA) pre-market ...
February 14, 2018 — The U.S. Food and Drug Administration (FDA) announced marketing clearance for Viz.AI’s Contact ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...

There have been several advancements in pacemaker technologies over the past few years. This is an overview of some of ...
Abbott announced the first patient has been enrolled in a clinical trial evaluating 28 days of dual antiplatelet therapy (DAPT) in patients at high risk of bleeding after implantation with a Xience everolimus-eluting coronary stent. The first patient was enrolled into the study by Prof. Emanuele Barbato, M.D., Ph.D., a cardiologist at OLV-Hospital Aalst in Belgium.

Clinical research has revealed men and women often have different presentations for cardiovascular disease (CVD). This ...


 February 19, 2018
February 19, 2018