Nemours Children’s Health System, a Florida-based health system with locations in six states, is now using in-house medical 3-D printing technology to create surgical models utilizing a U.S. Food and Drug Administration (FDA)-approved segmentation software. The software helps surgeons plan complex multidisciplinary cases in interventional radiology, cancer surgery and cardiac surgery. These models serve as a pre-planning blueprint and roadmap for Nemours surgeons and proceduralists, increasing confidence, reducing procedure times and minimizing unexpected findings while in the operating room.
Men under 50 who smoked were more likely to have a stroke, and their risk increased with the number of cigarettes they smoked, according to new research. The research was published in the American Heart Association’s journal Stroke.
Emerging medical device company Emboline Inc. announced it has completed a Series B funding round totaling over $10 million for its total embolic protection device for transcatheter aortic valve replacement (TAVR) procedures. The funding includes $3 million in new equity financing from multiple investors led by SV Tech Ventures and Shangbay Capital, and over $7 million in conversion of previously-issued convertible notes.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
A 360 degree view of the newest cath lab at Northwestern Medicine Central DuPage Hospital in Winfield, Ill., located in ...
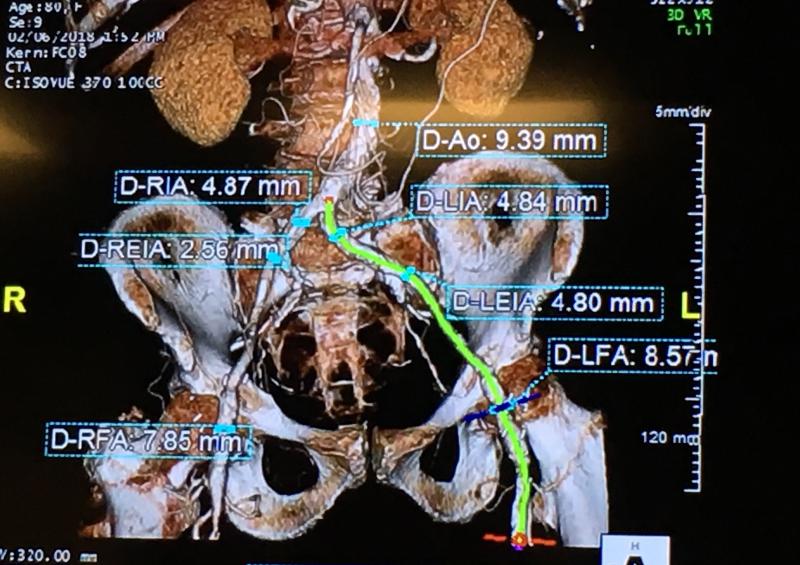
While there is fear by many about the trend of hospitals consolidating into larger healthcare systems, one advantage ...

Diagnostic and Interventional Cardiology was honored with a pair of Azbee Awards for editorial excellence at the 2018 Upper Midwest Regional banquet in Chicago, hosted by the American Society of Business Publication Editors (ASBPE).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
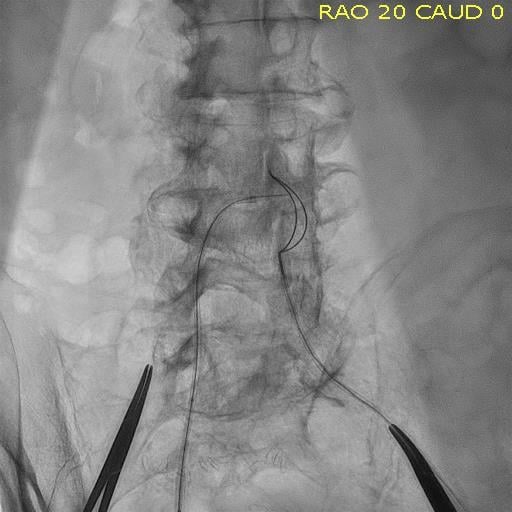
Patient selection for who is best suited for a transcatheter aortic valve replacement (TAVR) procedure is partly based ...
Many of the world’s leading interventional cardiologists and cardiovascular professionals will convene in San Diego, Calif., April 25-28, 2018, for the Society for Cardiovascular Angiography and Interventions (SCAI) 2018 Scientific Sessions. The annual event will attract nearly 2,000 leading interventionalists and cardiovascular professionals from across the globe for a dynamic four-day program featuring 12 live cases, late-breaking clinical science, case reviews, abstract presentations and interactive workshops.
April 18, 2018 — The U.S. Food and Drug Administration (FDA) has cleared the Somatom go.All and Somatom go.Top computed ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Northwestern Medicine has purchased several smaller Chicago suburban hospitals in the past few years to expand its ...
April 17, 2018 — The U.S. Food and Drug Administration (FDA) announced market approval for the Abbott Perclose ProGlide ...
Philips Healthcare last week issued a proactive advisory warning to its iSite and IntelliSpace picture archiving and communication system (PACS) customers of potential security vulnerabilities in the products. The company cautioned that while it has received no reports of patient harm, the vulnerabilities in question could impact or potentially compromise patient confidentiality, system integrity and/or system availability.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
The skin presents a formidable barrier to life-saving defibrillators, but a team of students from Rice University believes it has found a way around that problem.

Over the last decade, there have been considerable developments in procedural techniques and technology facilitating the ...
Medtronic plc announced U.S. Food and Drug Administration (FDA) approval to begin an investigational device exemption (IDE) pivotal trial to evaluate the Symplicity Spyral renal denervation system in patients with high blood pressure (hypertension). Renal denervation is a minimally invasive procedure intended to regulate the activity of nerves that lead to and from the kidney, which plays an important role in managing blood pressure. The SPYRAL HTN Pivotal Trial is part of the broader SPYRAL HTN Global Clinical Program, a multi-phased clinical study strategy aimed to establish the safety and efficacy of renal denervation to lower blood pressure.


 April 25, 2018
April 25, 2018