Mandatory public reporting of coronary artery bypass grafting (CABG) results in Massachusetts was associated with better patient outcomes compared to national findings, according to a recent study. Results of the 13-year Massachusetts experience were presented in a plenary session of the American Association for Thoracic Surgery’s 98th Annual Meeting.

The introduction of thermal ablation revolutionized the treatment of varicose veins, yet recurrence remains a stubborn ...
The acquisition of biomedical equipment company Esaote SpA’s share capital was completed on April 19, the company recently announced, by a consortium of leading Chinese investors. The consortium is composed of major companies in the medical and healthcare technology sectors, as well as investment funds with significant experience in this field.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Point of Care Decision Support (PCDS) announced the release of a new version 2.0 of their anticoagulation clinical decision support (CDS) software. The application was developed with leading clinical thrombosis experts to specifically address the new and complex challenges of managing both warfarin and non-vitamin K antagonist oral anticoagulants (NOACs). It enables healthcare provider teams to deliver consistent, measurable and evidence-based quality of care for patients on anticoagulants.
ITN Associate Editor Jeff Zagoudis explores how the mobile stroke unit (MSU) program at Northwestern Medicine Central ...
Physio-Control, now part of Stryker, announced its newest version of the LUCAS 3 Chest Compression System, version 3.1. The latest version provides new capabilities for tailored device functionality, wireless reporting and device status notifications sent over email.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
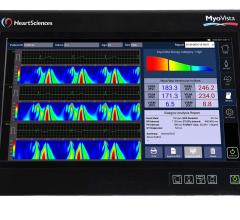
HeartSciences announced the results of a clinical study of its electrocardiography device that applies continuous wavelet transform signal processing and artificial intelligence. The study was serially conducted at The Icahn School of Medicine, Mount Sinai Hospital, New York, N.Y., and the West Virginia University (WVU) Heart and Vascular Institute, Morgantown, W. Va. The results were published online and in the April 17, 2018 issue of Journal of the American College of Cardiology (JACC).
April 27, 2018 — Intact Vascular Inc. recently closed a Series C financing totaling $20 million. This financing is ...
The European Patent Office (EPO) has revoked Edward Lifesciences’ Corp.’s European patent EP 2,399,550 (‘550), ruling in favor of Boston Scientific and several other companies involved in an ongoing dispute related to transcatheter heart valves. The ‘550 patent is also the sole basis of Edwards’ infringement action against Boston Scientific in Ireland.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

Over the past year, cardiac ultrasound vendors have introduced several new products to speed workflow, improve image ...

There have been a few big, recent advancements in cardiac computed tomography angiography (CCTA) imaging technology. The ...
Organizations who are embarking on an enterprise imaging journey share many of the same questions and concerns regarding ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
April 26, 2018 — Patients battling cardiovascular diseases, and even people hoping to prevent heart disease and stroke ...
HeartFlow Inc. announced that the National Health Service (NHS) England has chosen the HeartFlow FFRct (fractional flow reserve computed tomography) Analysis as part of the Innovation and Technology Payment (ITP) program. The HeartFlow Analysis was chosen as a new technology to be funded by ITP through a competitive process of nearly 300 applicants. It is the only ITP recipient focused on coronary artery disease (CAD), which affects 2.3 million people in the U.K., according to the British Heart Foundation.
The U.S. Food and Drug Administration (FDA) released a new Medical Device Safety Action Plan outlining how the agency will encourage innovation to improve safety, detect safety risks earlier, and keep doctors and patients better informed.

 April 30, 2018
April 30, 2018