May 9, 2018 — The U.S. Food and Drug Administration (FDA) said it recently granted market clearance for Angel Medical ...
May 9, 2018 — Stroke rehabilitation specialists at the The Ohio State University Wexner Medical Center are among the ...
A new clinical trial at The Ohio State University Wexner Medical Center is examining an implanted device that uses vagus ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Biosense Webster Inc. announced its Carto Vizigo Bi-directional Guiding Sheath is now available in the United States. According to the company, this is the first commercially available steerable guiding sheath that can be visualized on the Carto 3 System during a catheter ablation procedure, helping electrophysiologists (EPs) reduce dependency on fluoroscopy.
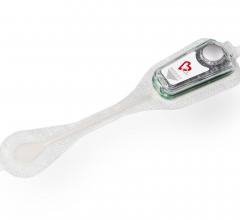
Bardy Diagnostics Inc. (BardyDx) announced that the American Heart Journal has published the results of a head-to-head comparison of two patch-based arrhythmia monitoring systems. The study, "Comparison of two ambulatory patch ECG monitors: The benefit of the P-wave and signal clarity," concluded the BardyDx CAM single-channel patch ambulatory ECG monitor (AEM), designed specifically to enhance P-wave detection, identified significantly more arrhythmias and resulted in better, more informed clinical decision-making over the iRhythm Zio XT patch.
May 8, 2018 — One-year results from the Sentinel Cerebral Protection System show it can reduce the incidence of stroke ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
A new data analysis of the Northwestern Medicine Mobile Stroke Unit (MSU) found the specialized ambulance provided life-saving treatment 30 minutes faster than traditional transport in its first year of operation. The analysis found, on average, the MSU delivered the clot-busting drug tPA to ischemic stroke patients 52 minutes after 9-1-1 dispatch, compared to an average of 82 minutes for patients transported via ambulance.
Colibri Heart Valve LLC announced the Colibri transcatheter aortic valve implantation (TAVI) System has been used on an additional four patients in the ongoing international, single-arm, open-label early feasibility study (EFS). Initial post-implantation results from these patients, who received a 24mm valve, show favorable low aortic valve pressure gradients and no observed paravalvular leakage or aortic insufficiency. The Colibri TAVI System is being developed with 21-, 24-, 27- and 30mm valves to accommodate a variety of clinical and patient needs.
The U.S. Food and Drug Administration (FDA) has qualified the Minnesota Living with Heart Failure Questionnaire from the University of Minnesota as part of the Medical Device Development Tools (MDDT) program. This voluntary program is intended to reduce regulatory burden for medical device developers and FDA reviewers by qualifying tools that can aid in the development and evaluation of medical devices. Tools qualified by the FDA can be used by the medical device industry to support device submissions, which could reduce time and resources involved in product development.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

The European interventional cardiology market is currently valued at nearly $1.4 billion. This is a mature market that ...
Imran Ahmad, M.D., medical director of interventional cardiology, explains some of the new technologies his labs have ...

In many cases, the diagnosis and management of patients with rare diseases can require the participation of ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...

The U.S. Food and Drug Administration (FDA) has approved Portola Pharmaceuticals' Andexxa, the first antidote indicated for patients treated with rivaroxaban (Xarelto) and apixaban (Eliquis), when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.
Stereotaxis and Acutus Medical announced a strategic collaboration to integrate the Stereotaxis Niobe Magnetic Navigation System and the Acutus Medical AcQMap High Resolution Imaging and Mapping System. The goal of the collaboration is to improve patient care and the physician experience in electrophysiology.
In March, I made a day trip to New York City to receive the 2017 Jesse H. Neal Award for Best Use of Social Media. The ...


 May 09, 2018
May 09, 2018