Abbott announced U.S. Food and Drug Administration (FDA) approval of the TactiCath Contact Force Ablation Catheter, Sensor Enabled, a new ablation catheter designed to help physicians accurately and effectively treat atrial fibrillation (AFib). The approval further expands Abbott's portfolio of cardiac ablation tools that integrate with the company's EnSite Precision cardiac mapping system to help physicians develop more precise images of the heart during cardiac ablation procedures.
Philips announced the launch of Azurion with FlexArm, designed to enhance positioning flexibility for image-guided procedures.

Many of the latest advances in cardiovascular imaging technologies are unveiled each year at the Radiological Society of ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The U.S. Food and Drug Administration (FDA) issued a letter Jan. 17, 2019, to healthcare providers regarding a recent publication that suggests a possible increased risk of death at two years and beyond in patients treated for a type of peripheral artery disease (PAD) with paclitaxel-coated balloons or paclitaxel-eluting stents. The letter was issued in response to a recent publication in the Journal of the American Heart Association identifying the risk. It recommends doctors continue to monitor these patients, and discuss the benefits and risks of all available treatment options for patients with PAD.

Artificial intelligence (AI) was by far the hottest trend discussed in sessions and across the expo floor at the world's ...
Philips announced that NewYork-Presbyterian Hospital has chosen to implement the company’s IntelliSpace Enterprise Edition solution across its hospitals and facilities. In a 10-year agreement, IntelliSpace Enterprise Edition will provide the health system with access to Philips’ full suite of informatics solutions across radiology, cardiology and analytics to help improve efficiencies and care delivery.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Cerenovus, of Johnson & Johnson Medical Devices Companies, recently launched the EXCELLENT Registry to collect and analyze stroke-inducing blood clots removed from the brain with its Embotrap II Revascularization Device. The company also announced European CE Mark approval for the Geometric Clot Extractor (GCE) Revascularization Device.
Shockwave Medical Inc. has initiated its U.S. Food and Drug Administration (FDA) Investigational Device Exemption (IDE) study – DISRUPT CAD III – for the use of intravascular lithotripsy (IVL) in heavily calcified coronary arteries. IVL is a lesion preparation tool designed to fracture problematic calcium using sonic pressure waves in order to facilitate stent delivery, deployment and expansion.
Abbott announced the U.S. Food and Drug Administration (FDA) approved the Amplatzer Piccolo Occluder to treat patent ductus arteriosus (PDA). The company calls it the world's first medical device that can be implanted in the tiniest babies (weighing as little as two pounds) using a minimally invasive procedure to treat PDA. The Amplatzer Piccolo, a device even smaller than a small pea, now offers hope to premature infants and newborns who need corrective treatment, and who may be non-responsive to medical management and high risk to undergo corrective surgery.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Boston Scientific Corp. and Edwards Lifesciences Corp. announced that the companies have reached an agreement to settle all outstanding patent disputes between the companies in all venues around the world. All pending cases or appeals in courts and patent offices between the two companies will be dismissed, and the parties will not litigate patent disputes related to current portfolios of transcatheter aortic valves, certain mitral valve repair devices, and left atrial appendage closure devices. Any injunctions currently in place will be lifted.
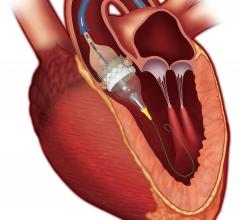
Mitral regurgitation (MR) is one of the most common types of heart valve diseases in the United States, affecting ...
In an age when everything in medicine is now looked at though a cost vs. benefit analysis and U.S. government healthcare ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

Here are a few of the takeaways from the clinical studies presented and new technology shown on the exhibit floor at the ...

To extract or abandon broken or infected implantable, venous electrophysiology (EP) device leads has been a debate for ...
Research suggests that a gene that governs the body’s biological (circadian) clock acts differently in males versus females and may protect females from heart disease. The study is the first to analyze circadian blood pressure rhythms in female mice. The research was published ahead of print in the American Journal of Physiology — Regulatory, Integrative and Comparative Physiology.


 January 21, 2019
January 21, 2019