At the recent Healthcare Information and Management Systems Society (HIMSS) annual conference, AT&T’s healthcare team demonstrated the key role connectivity and the internet of medical things (IoMT) plays throughout the continuum of care. This concept was highlighted by a demonstration of the Bodyport remote monitoring solution.
Navitian, the new coronary microcatheter from iVascular, recently received CE mark approval. The device was approved to facilitate, guide and support a guidewire while accessing the coronary system, the exchange of guidewires, and injection of radiopaque contrast media or saline solutions.
February 20, 2019 — Post-traumatic stress disorder (PTSD) by itself does not explain the increased risk of ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Medtronic is recalling its dual chamber implantable pulse generators (IPGs) due to the possibility of a software error that can result in a lack of pacing. Patients and physicians cannot predict whether and when this software error might occur. A lack of pacing could result in patients experiencing slow heart beating, low blood pressure, and symptoms such as light headedness, fainting and even death.
Clinical study data makes the world go around in cardiology and is the basis of setting guidelines in evidence-based ...
California-based Vascular Dynamics Inc. (VDI) is sponsoring a new clinical trial, called CALM-2 (Controlling And Lowering blood pressure with MobiusHD), of a novel endovascular approach to treat patients with drug-resistant hypertension. St. Bartholomew’s Hospital in London is helping to lead efforts in the U.K. as a key study site.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Ochsner Health System in Louisiana recently announced a new partnership with device data management and remote monitoring vendor Geneva Health Solutions (GHS) for implantable cardiac devices. This partnership marks the first time the GHS cloud-based technology platform and remote monitoring service for patients with cardiac implants will be utilized in the region, which includes Louisiana, Arkansas, Mississippi, Alabama and Georgia. The GHS system will drive full automated scheduling and reporting in collaboration with Ochsner’s established Epic electronic medical record (EMR).
A new reversible, drug-free antiplatelet therapy could reduce the risk of blood clots and potentially prevent cancer metastasis, according to a study published in Science Translational Medicine.
Patients receiving hormone therapy as part of their gender-transition treatment had an elevated risk for cardiovascular events, including strokes, heart attacks and blood clots, according to a study published in the American Heart Association’s journal Circulation.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Foldax Inc. announced the U.S. Food and Drug Administration (FDA) has granted investigative device exemption (IDE) approval for an Early Feasibility Study of the Tria surgical aortic heart valve to treat aortic valve disease.
Philips announced the launch of Philips Zenition, its new mobile C-arm imaging platform. Mobile C-arms are X-ray systems that are brought into the operating room (OR) to provide live image guidance during a wide range of surgeries including orthopedic, trauma and vascular procedures. The Zenition mobile C-arm platform brings together innovations in image capture, image processing, ease-of-use and versatility pioneered on Philips’ Azurion platform. Zenition allows hospitals to maximize OR performance, enhance their clinical capabilities and offer their staff a high-quality user experience. Zenition will be introduced in the U.S., Germany, Austria and Switzerland in the first half of 2019, and will subsequently be rolled out in further markets.
Epsilon Imaging recently launched the new EchoInsight Pro Starter configuration for those programs that may be budget constrained or just getting started with the incorporation of strain imaging into echo studies. The EchoInsight Pro Starter configuration starts with a two-seat bundle at an affordable, discounted package price, and can scale to up to six client seats.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
IBM Watson Health and the Broad Institute of MIT and Harvard are launching a research partnership aimed at developing powerful predictive models that will enable clinicians to identify patients at serious risk for cardiovascular disease. This three-year project will incorporate population- and hospital-based biobank data, genomic information, and electronic health records to build upon and expand the predictive power of polygenic scoring. The project will also plan to make insights and tools widely available to the research community, including methods to calculate an individual’s risk of developing common diseases based on millions of variants in the genome.
Corindus Vascular Robotics Inc. is seeking premarket clearance from the U.S. Food and Drug Administration (FDA) to use the CorPath GRX System in neurovascular intervention. CorPath GRX, the second-generation robotic platform, received FDA clearance for percutaneous coronary intervention (PCI) in 2016 and peripheral vascular intervention (PVI) in 2018. Upon successful FDA clearance, CorPath GRX would become the world’s first and only robotic platform indicated for use in PCI, PVI and neurovascular intervention (NVI).
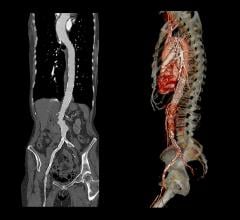
Abdominal aortic stent grafts will remain the largest segment in the global aortic stent graft market at least through 2028 due to the high prevalence of abdominal aortic aneurysms (AAA) relative to thoracic abdominal aneurysms (TAA), according to a new report from GlobalData. The abdominal segment will reach an estimated $2.8 billion global value by 2028, while the overall global aortic stent graft market is expected to reach $4.5 billion in the same period. This represents a compound annual growth rate of 5.4 percent between 2018 and 2028.


 February 21, 2019
February 21, 2019