May 13, 2019 – A first-in-human pilot study of Medtronic's investigational Extravascular Implantable Cardioverter ...
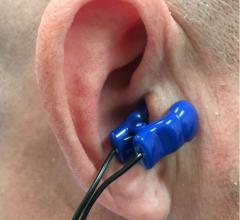
May 13, 2019 — A randomized clinical trial effectively used nerve stimulation through an ear clip to reduce atrial ...
May 13, 2019 — A digital screening for atrial fibrillation (AFib or AF) successfully monitored more than 60,000 ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
BioTrace Medical Inc. announced the company’s Tempo Lead has obtained CE Mark certification in Europe for use in cardiac procedures requiring temporary intracardiac pacing.
AliveCor announced its third U.S. Food and Drug Administration (FDA) clearance in three months, making KardiaMobile 6L the world's first available six-lead personal electrocardiogram (ECG) device, according to the company. This clearance gives patients and their physicians an even more detailed view into patients' hearts, including visibility into certain arrhythmias that are leading indicators of cardiovascular disease.
Imricor announced the signing of a commercial agreement with the Haga Hospital in The Hague, Netherlands to outfit a magnetic resonance imaging (MRI) ablation center for treating cardiac arrhythmias.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The integration of artificial intelligence (AI) into medicine has by far been the hottest topic at nearly all medical ...
Shine Medical Technologies Inc. broke ground on their first medical isotope production facility in Janesville, Wis. U.S. Department of Energy (DOE) Under Secretary for Nuclear Security and Administrator of the National Nuclear Security Administration (NNSA) Lisa Gordon-Hagerty, Lantheus Medical Imaging President and CEO Mary Heino and Janesville City Manager Mark Freitag joined Shine founder and CEO, Greg Piefer, and the Shine team to celebrate the milestone.
Spacelabs Healthcare debuted Sentinel 11 at the 40th Annual Heart Rhythm Society (HRS) Scientific Sessions, May 8-11, 2019, in San Francisco. This latest release from Spacelabs advances cardiology information management with optimized workflows, expanded connectivity and robust data security capabilities.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

May 10, 2019 — Here is the list of the late-breaking hot line studies being presented at the 2019 EuroPCR interventional ...
May 10, 2019 — Diagnostic and Interventional Cardiology (DAIC) earned a Bronze Award at the 2019 National Azbee Awards ...
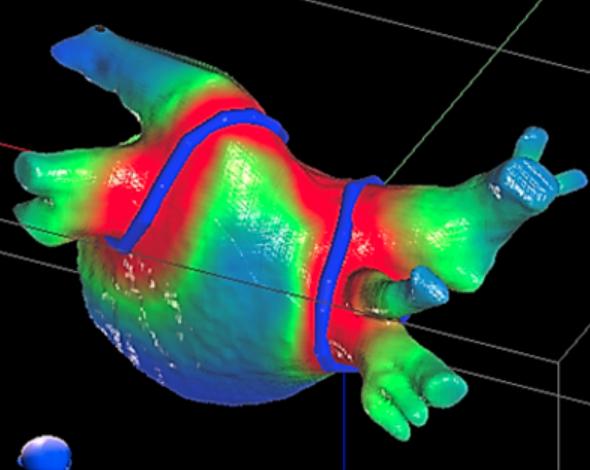
May 10, 2019 — Radiation oncology vendor Varian announced it acquired the start-up company CyberHeart, which has ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Osprey Medical announced the launch of DyeMINISH, a global patient registry to evaluate the ongoing safety and performance of the DyeVert Contrast Reduction System during standard clinical use in a real-world patient population. The DyeMINISH registry is a retrospective, large-scale, multi-center study, with the first patient included on May 7, 2019. The registry is designed to enroll up to 10,000 participants and is expected to complete in late 2023.
Stereotaxis introduced Stereotaxis Genesis RMN, its next-generation robotic platform that it claims is a significant advancement in robotic magnetic navigation technology.
Philips announced a collaboration with Medtronic to further advance treatment of paroxysmal atrial fibrillation (PAF), a common heart rhythm disorder. Through the agreement, Medtronic will facilitate sales of products on behalf of Philips to provide an integrated image guidance solution for cryoablation procedures. Philips will bring to market the novel KODEX-EPD cardiac imaging and navigation system with cryoablation specific features to enable electrophysiologists to perform cryoablation procedures with reduced need for X-ray imaging.


 May 13, 2019
May 13, 2019