December 12, 2019 — Cardiologs, a global leader in artificial intelligence (AI) cardiology diagnostics, announced today ...
December 9, 2019 — DiA Imaging Analysis Ltd., an IBM Alpha Zone Accelerator Alumni Startup, announces a collaboration ...
November 27, 2019 — CAE Healthcare will showcase its mixed reality training solutions for practicing physicians and ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
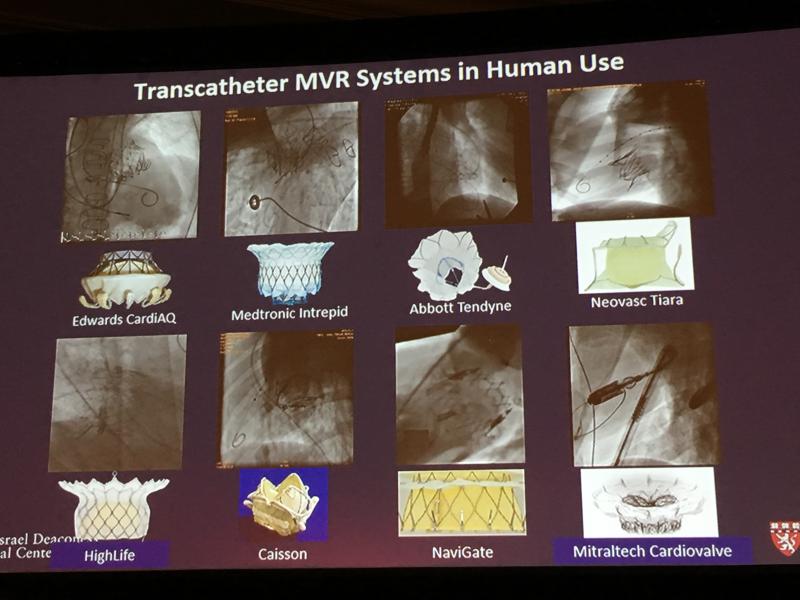
The overwhelming success story for transcatheter aortic valve replacement (TAVR) moving from a science project to ...

November 26, 2019 — Physicians from the University of New Mexico (UNM) and local emergency responders recently treated a ...
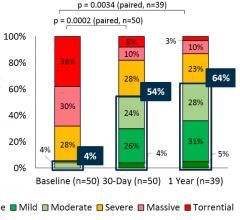
November 26, 2019 — The preliminary one-year results of the TRILUMINATE Pivotal Study for Abbott's TriClip device, a ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
November 26, 2019 — The University of Connecticut (UConn) Department of Kinesiology and Hartford Healthcare have ...
The U.S. Food and Drug Administration (FDA) has cleared the Medtronic In.Pact AV drug-coated balloon (DCB) for the treatment of failing arteriovenous (AV) access in patients with end-stage renal disease (ESRD) undergoing dialysis.
November 22, 2019 — Artificial intelligence can examine electrocardiogram (ECG) test results, a common medical test, to ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Now, more than ever, the field of cardiology needs women. But as the national need for more cardiologists overall ...

There were a few key takeaways from the American Society of Nuclear Cardiology (ASNC) 2019 annual meeting in September, these included...

Interest in distal radial artery access (DRA) is growing rapidly. Among the benefits of DRA are the low risk of entry ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
November 21, 2019 — At the American Heart Association Scientific Sessions 2019, Eko, a digital health company applying ...
November 21, 2019 — People who experience cardiac arrests over the weekend are less likely to survive long enough to be ...
November 21, 2019 — Endotronix, a digital health and medical technology company dedicated to advancing the treatment of ...


 December 12, 2019
December 12, 2019