vRad (Virtual Radiologic), a Mednax company recently made a scientific presentation, “Screening for Aortic Dissection on CT Angiography Using a Convolutional Neural Network,” at the Society for Imaging Informatics in Medicine (SIIM) Annual Meeting, June 26-28 in Aurora, Colo.
Catheter Precision Inc. announced that the U.S. Food and Drug Administration (FDA) has cleared its new VIVO (View into Ventricular Onset) system for market release in the United States. VIVO is a pre-procedure planning tool that offers 3-D cardiac mapping to aid in localizing the sites of origin of idiopathic ventricular arrhythmias in patients with structurally normal hearts prior to electrophysiology studies. The novel VIVO system uses magnetic resonance imaging (MRI) or a computed tomography (CT) scan along with a standard 12-lead ΕCG to computer-generate color-coded 3-D mapping images of the heart to indicate the area of earliest activation.
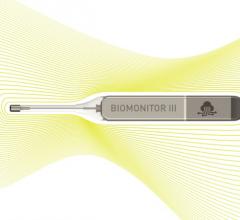
Biotronik announced the market release of its new injectable cardiac monitor (ICM), Biomonitor III, following approval in the CE region. The novel device is designed to help patients with irregular heart rhythms by documenting suspected arrhythmia or unexplained syncope with increased clarity.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Federico Asch, M.D., FASE, director of cardiac imaging research and director of the cardiovascular imaging lab, MedStar ...
Partho Sengupta, M.D., MBBS, chief of cardiology, West Virginia Heart and Vascular Institute, explains how wearable ...
Sharon Mulvagh, M.D., FASE, FACC, FRCPC, professor of medicine, division of cardiology, Dalhousie University, Halifax ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
This is a quick example of how artificial intelligence (AI) is being integrated on the back end of cardiac ultrasound ...
Marielle Scherrer-Crosbie, M.D., Ph.D., director of echocardiography at the Hospital of the University of Pennsylvania ...
Federico Asch, M.D., FASE, director of cardiac imaging research and director of the cardiovascular imaging lab, MedStar ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Judy Hung, M.D., director of echocardiography, Division of Cardiology, Massachusetts General Hospital, Boston, explains ...
Roberto Lang, M.D., director of cardiac imaging at the University of Chicago, has been working with TomTec for the past ...
The U.S. Food and Drug Administration (FDA) issued the final guidance: Marketing Clearance of Diagnostic Ultrasound Systems and Transducers. This final guidance provides detailed recommendations for manufacturers seeking marketing clearance of diagnostic ultrasound systems and transducers.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Thomas Porter, M.D., FASE, the Theordore F. Hubbard Distinguished Chair of Cardiology and a professor of medicine at the ...
Siemens Healthineers and Mentice AB announced the collaboration to fully integrate Mentice’s VIST Virtual Patient into the Artis icono angiography system from Siemens Healthineers. The VIST Virtual Patient thus becomes a fully integrated simulation solution for the angio suite, according to the companies. The global partnership between the two companies will allow interventional radiologists, neuroradiologists, and cardiologists to perform vascular and cardiac interventions on a virtual patient inside the angio-suite.
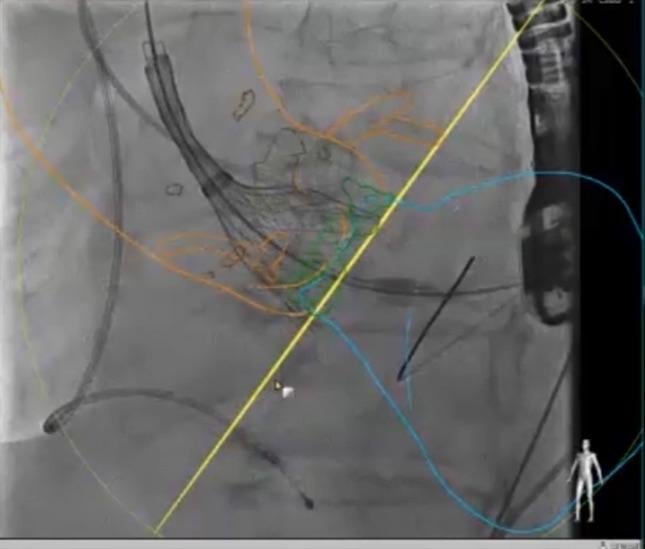
The Centers for Medicare and Medicaid Services (CMS) recently finalized its decision to update the national coverage policy for transcatheter aortic valve replacement (TAVR), streamlining key elements of the original national coverage determination that went into effect in 2012.

 July 01, 2019
July 01, 2019