People suffering from insomnia may have an increased risk of coronary artery disease, heart failure and stroke, according to new research in the American Heart Association’s journal Circulation.
The U.S. Food and Drug Administration (FDA) approved the Barostim Neo System for the improvement of symptoms in patients with advanced heart failure who are not suited for treatment with other heart failure devices, such as cardiac resynchronization therapy. The FDA gave the device a Breakthrough Device designation because it treats a life-threatening disease, heart failure, and addresses an unmet medical need in patients who fail to get adequate benefits from standard treatments and have no alternative treatment options.
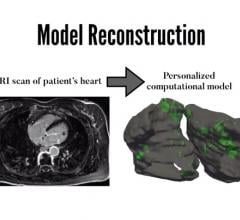
Scientists at Johns Hopkins have successfully created personalized digital replicas of the upper chambers of the heart and used them to guide the precise treatment of patients suffering from persistent irregular heartbeats. These simulations accurately identified where clinicians need to destroy tissue to restore the heart’s normal rhythm.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Every second counts for stroke patients, as studies show they can lose up to 27 million brain cells per minute. Researchers at The University of Texas Health Science Center at Houston (UTHealth) recently published new findings in Stroke that show patients transported to the hospital by mobile stroke unit (MSU) instead of standard ambulance received a clot-busting procedure an average of 10 minutes faster, which could potentially save up to 270 million neurons per patient.
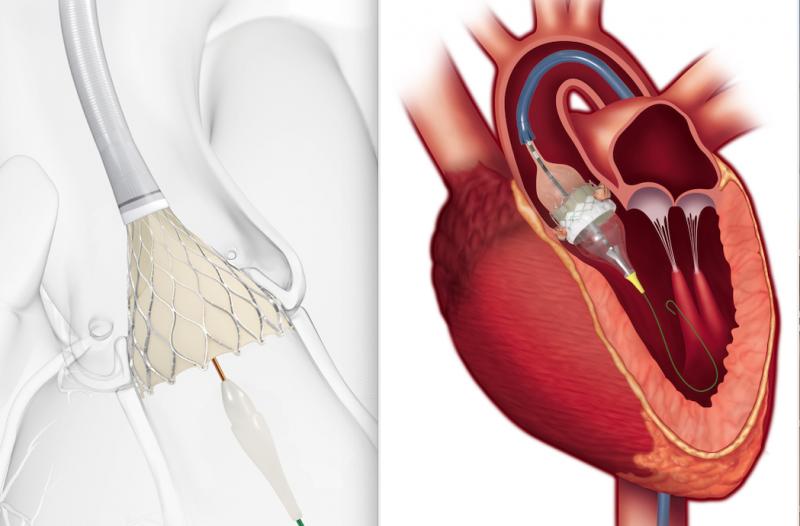
In one coordinated move, the U.S. Food and Drug Administration (FDA) opened transcatheter aortic valve replacement (TAVR) to low-risk patients with the simultaneous approvals of Edwards Lifesciences’ Sapien 3 valve and Medtronic’s CoreValve Evolut system for this critical patient population. The low-risk patient population is the final surgical risk category to be approved for TAVR, a minimally invasive alternative to open-heart surgical valve replacement (SAVR), and includes patients who may be younger and more active than higher-risk patients. Both devices are indicated for patients with severe, symptomatic aortic stenosis (AS).
Bardy Diagnostics Inc. announced that HealthTech Arkansas, a healthcare accelerator and investment fund that connects early-stage healthcare companies with disruptive technologies to Arkansas healthcare providers, has selected BardyDx to participate in the organization's 2019 accelerator program. BardyDx was chosen for its advancements in cardiac monitoring by delivering diagnostic accuracy with the Carnation Ambulatory Monitor (CAM), a P-wave centric ambulatory cardiac patch monitor and arrhythmia detection device.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Thin, flexible fibers made of carbon nanotubes have now proven able to bridge damaged heart tissues and deliver the electrical signals needed to keep those hearts beating.
People enrolled in a large clinical hypertension management trial were half as likely to control their blood pressure if they received care at clinics and primary care practices in low-income areas, according to new research in Journal of the American Heart Association.
Ancora Heart Inc. announced the first patient was enrolled in the CorCinch EU study, a European multi-center clinical evaluation of the AccuCinch Ventricular Repair System as a treatment for patients with reduced ejection fraction systolic heart failure (HFrEF).
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Concept Medical Inc. (CMI) has been granted "Breakthrough Device Designation" from the U.S. Food and Drug Administration (FDA) for MagicTouch PTA, its sirolimus drug coated balloon (DCB) catheter, for the treatment of peripheral artery disease (PAD) in below-the-knee (BTK) applications.
A simple change in the way health professionals track their patients’ progress has brought improved healing of life-altering open sores caused by chronic venous insufficiency (CVI) , according to a new article in the Journal for Vascular Surgery – Venous and Lymphatic Disorders.
Non-invasive techniques and devices for assessing blood flow and other diagnostic considerations for people with critical limb ischemia are addressed in a new scientific statement from the American Heart Association. The statement is published in the association’s flagship journal Circulation.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
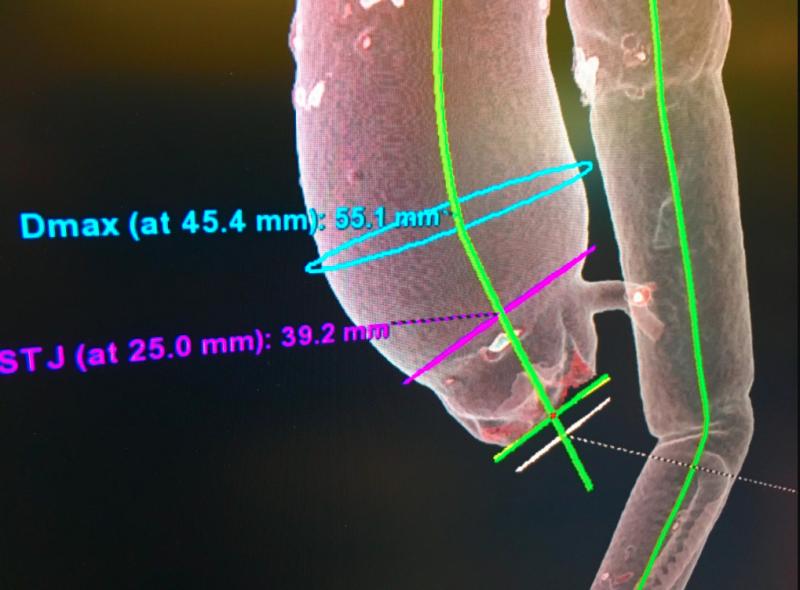
As we usher in a new era for treating patients with aortic stenosis, we have been reflecting on the evolution of the ...
You have likely heard the case for moving to structured reporting, including standardized documentation to drive more ...
An example of Siemens' photo-realistic Cinematic image reconstruction. This image is from a CTA exam from a Siemens ...


 August 19, 2019
August 19, 2019