This is an example of an augmented reality (AR) training system for transesophageal echo (TEE) created by the simulation ...
December 19, 2019 — The U.S. Food and Drug Administration (FDA) has granted breakthrough status for a novel ECG-based ...
DAIC Editor Dave Fornell and Imaging Technology News (ITN) Consulting Editor Greg Freiherr offer a post-game report on ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
December 18, 2019 — In their latest report, “Cardiovascular Disease 2020-2030: Trends, Technologies & Outlook” IDTechEx ...
December 18, 2019 — Append Medical, developer of a novel left atrial appendage (LAA) closure device to minimize stroke ...
December 18, 2019 — BioVentrix, Inc., developer of the first less invasive system for left ventricular remodeling, today ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
December 18, 2019 — Cook Medical initiated a recall of its CrossCath Support Catheters in November, which the U.S. Food ...

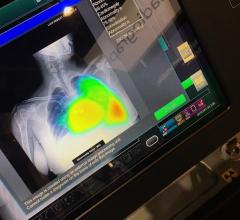
December 16, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...

The U.S. Food and Drug Administration (FDA) approved the use of Vascepa (icosapent ethyl) capsules as an adjunctive therapy to reduce the risk of cardiovascular events in adults with elevated triglyceride levels
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
December 12, 2019 — Impulse Dynamics, developer of Optimizer Smart System for delivering CCM therapy, announced the ...
December 12, 2019 — Low-dose aspirin was not associated with a reduced risk of a fatal heart attack among African ...
December 12, 2019 — Cardiologs, a global leader in artificial intelligence (AI) cardiology diagnostics, announced today ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
December 9, 2019 — DiA Imaging Analysis Ltd., an IBM Alpha Zone Accelerator Alumni Startup, announces a collaboration ...
November 27, 2019 — CAE Healthcare will showcase its mixed reality training solutions for practicing physicians and ...
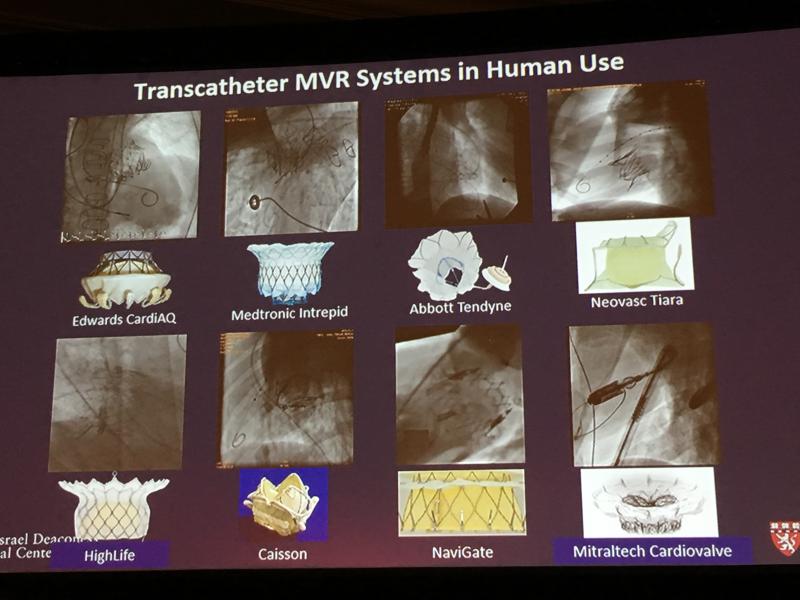
The overwhelming success story for transcatheter aortic valve replacement (TAVR) moving from a science project to ...


 December 19, 2019
December 19, 2019