September 4, 2019 – Novartis announced results from two new clinical trials evaluating improvement in heart structure ...
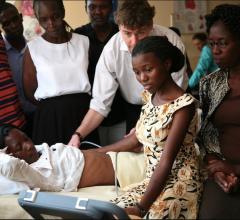
September 4, 2019 — Final data from the World Alliance Societies of Echocardiography (WASE) Normal Values Study was ...
Shockwave Medical Inc. has received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for its Shockwave intravascular lithotripsy (IVL) system with the Shockwave C2 Coronary IVL Catheter. The device is currently the subject of an Investigational Device Exemption (IDE) study called DISRUPT CAD III. The Shockwave C2 IVL Catheter is a proprietary tool designed to fracture problematic calcium using sonic pressure waves in order to facilitate stent delivery, deployment and optimal expansion, thereby improving blood flow to the heart muscle.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Corindus Vascular Robotics Inc. announced that EClinicalMedicine, a clinical journal published by The Lancet, has published the results from the Telerobotic Intervention Study, the world’s first percutaneous coronary intervention (PCI) procedures conducted from a remote location outside the catheterization lab. The study was conducted using Corindus’ CorPath technology platform.
New Zealand-based Adept Medical announced the launch of the Antegrade IR Platform. Clinically driven, it is placed to provide an ideal work surface for antegrade femoral approach during interventional radiology vascular procedures. The device sits within the company’s existing range of access and patient positioning devices designed for interventional radiology, cardiology and vascular procedures.

September 3, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Philips will showcase its latest cardiac care innovations at the European Society of Cardiology (ESC) Congress 2019, Aug. 31–Sept. 4 in Paris, France. At the congress, Philips is showcasing Release 5.0 of its Epiq CVx cardiology platform for the first time in Europe. The platform includes automated applications for 2-D assessment of the heart, as well as robust 3-D right ventricle volume and ejection fraction measurements, making accurate exams faster and easier to conduct. Philips also announced that it is collaborating with digital health company LindaCare to combine the latter’s OnePulse cloud-based solution for the remote monitoring of patients with cardiac implantable electronic devices (CIEDs) with the Philips IntelliSpace Cardiovascular informatics platform.

Until recently, cardiologists trying to diagnose and treat arrhythmias have had to deal with technological limitations ...
Use of the Internet of Things (IoT) is booming, with IHS Markit forecasting there will be 73 billion connected devices in use around the world by 2025. IoT technology has moved beyond speakers and smart fridges and is increasingly being utilized for critical applications across the healthcare industry like insulin delivery devices, connected inhalers and even cancer treatments.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
In the near future, doctors may be able to apply artificial intelligence (AI) to electrocardiogram data in order to measure overall health status, according to new research. The study was published in Circulation: Arrhythmia and Electrophysiology, a journal of the American Heart Association.
Atrial fibrillation, or Afib, kills about 130,000 people worldwide every year. It also affects 3 to 6 million people in ...
Merit Medical Systems Inc. announced the U.S. commercial launch of the PreludeSync Evo radial compression device. The PreludeSync Evo is a sterile, single-use, disposable device used to assist in gaining hemostasis of the arterial percutaneous access site following catheterization procedures.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...

The U.S. Food and Drug Administration (FDA) released an updated MedWatch Alert this month on the safety of paclitaxel-coated devices used to treat peripheral artery disease (PAD) following its review of long-term follow-up clinical data. The agency said five-year results from three randomized trials showed an increased mortality rate in patients treated with these devices compared to those treated with uncoated devices. While these data provide reason for caution, the FDA noted that the devices still provide documented short-term benefits, and healthcare providers should consider all options for their PAD patients.
AtriCure Inc. announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance of additional labeling claims for AtriClip left atrial appendage (LAA) management devices. These include changing the indication from occlusion of the LAA to exclusion, and also adding electrical isolation as a labeling claim.
A new 3-D magnetic resonance imaging (MRI) computing technique developed by scientists in WMG at the University of Warwick focuses on hierarchical template matching (HTM) to diagnose cardiac disease without the use of gadolinium contrast. The technique is explored in an article in the journal Scientific Reports.


 September 04, 2019
September 04, 2019