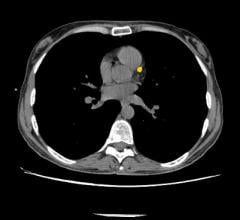
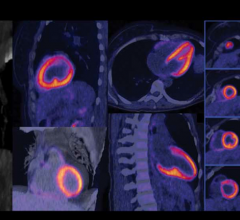
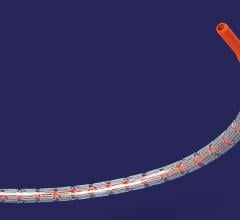
W. L. Gore & Associates Inc. (Gore) announced U.S. Food and Drug Administration (FDA) 510(k…
August 22, 2018 — Philips recently announced the introduction of the Epiq CVx …
iBeat recently announced the release and shipping of its iBeat Heart Watch. The heart and blood…
BioSig Technologies Inc. announced that the company received 510(k) clearance for its first…
July 25, 2018 — The U.S.
July 23, 2018 — The U.S.
July 18, 2018 — Abbott announced it received approval from the U.S.
Medtronic plc recently received U.S. Food and Drug Administration (FDA) approval for a less-…
Zebra Medical Vision has received 510(k) clearance from the U.S. Food and Drug Administration (…
July 10, 2018 — Evanston, Ill.-based startup Visura Technologies has received 510(k) clearance…
South Korea-based Healcerion launched the Sonon 300L wireless handheld ultrasound device to the…
Embolx Inc. has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for…
Dictum Health Inc., creators of the Virtual Exam Room (VER) telehealth technology, announced the…
Siemens Healthineers will announce U.S. Food and Drug Administration (FDA) clearance of four new…
Cardiovascular imaging artificial intelligence (AI) company Bay Labs announced its EchoMD AutoEF…
3D Systems announced availability of its new On Demand Anatomical Modeling Service. This new…
LivaNova PLC announced it received U.S. Food and Drug Administration (FDA) 510(k) clearance for…
Medtronic plc has received U.S. Food and Drug Administration (FDA) approval for 200mm and 250mm…
June 5, 2018 — The U.S.
Abbott announced it received approval from the U.S. Food and Drug Administration (FDA) for…


 August 23, 2018
August 23, 2018