Philips announced the launch of Azurion with FlexArm, designed to enhance positioning…
Edwards Lifesciences Corp. announced that the Sapien 3 Ultra system has received U.S. Food and…
CathWorks announced that its FFRangio System received U.S. Food and Drug Administration (FDA)…
Cardiva Medical Inc. announced the company has received premarket approval (PMA) from the U.S.…
Subtle Medical announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) to…
Siemens Healthineers announced the U.S. Food and Drug Administration (FDA) clearance of the Cios…
Digital health company Planet Intelligent recently launched the ExMedicus Smartwatch, what it…
October 17, 2018 — The U.S.
October 10, 2018 — Medical coding software provider ZHealth recently unveiled Etch, the first-…
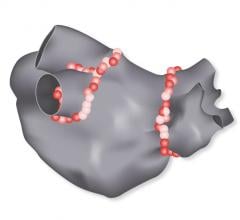
Biosense Webster Inc. recently received approval from the U.S. Food and Drug Administration (FDA…
The U.S. Food and Drug Administration (FDA) has cleared the Magnetom Sola, a 1.5 Tesla magnetic…
The U.S. Food and Drug Administration (FDA) granted market clearance for FibriCheck, a Belgian…
Veryan Medical Ltd has received Premarket Approval (PMA) for the BioMimics 3D Vascular Stent…
Fujifilm SonoSite Inc. announced its entry into the medical informatics space with the launch of…
Three-dimensional printing company Biomodex, which develops organ twins for advanced physician…
Boston Scientific announced that the U.S. Food and Drug Administration (FDA) has approved its…
September 27, 2018 — Biotronik recently announced U.S.
September 26, 2018 — Bruker recently announced the introduction of the new preclinical…
September 19, 2018 — DiA Imaging Analysis Ltd.


 January 17, 2019
January 17, 2019