Zebra Medical Vision announced it has received its third U.S. Food and Drug Administration (FDA…
Philips announced the U.S. Food and Drug Administration (FDA) has approved the company’s…
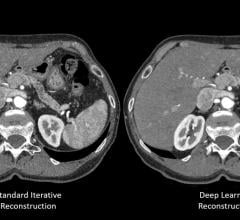
Canon Medical Systems USA Inc. has received 510(k) clearance on its new deep convolutional…
Three-dimensional (3-D) printing software and solutions company Materialise has received U.S.…
W. L. Gore & Associates (Gore) announced the U.S. Food and Drug Administration’s (FDA’s)…
Medivis announced that its augmented reality (AR) technology platform for surgical applications…
Medis Medical Imaging Systems B.V. has received clearance from the U.S. Food and Drug…
ControlRad Inc. announced that the U.S. Food and Drug Administration (FDA) granted 510(k)…
Medtronic plc announced its entrance into the guide extension catheter market with the global…
Medical diagnostic artificial intelligence (AI) company MaxQ AI announced that Accipio Ax will…
Philips announced the launch of the new IntraSight interventional applications platform. The…
May 17, 2019 — The U.S.
Digital healthcare company Murj announced the availability of the Murj Analytics software-as-a-…
Johnson & Johnson Medical Devices Companies announced the launch of Biosense Webster Inc.’s…
BioCardia Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the Avance…
W. L. Gore & Associates Inc. (Gore) announced the U.S. Food and Drug Administration (FDA)…
Cordis, a Cardinal Health company, recently announced the full U.S. launch of its Radial 360…
Artificial intelligence (AI) solutions provider Aidoc has been granted U.S. Food and Drug…
The U.S. Food and Drug Administration (FDA) has approved the expansion of Abiomed’s Impella 5.0…
AliveCor announced its third U.S. Food and Drug Administration (FDA) clearance in three months,…

 June 19, 2019
June 19, 2019