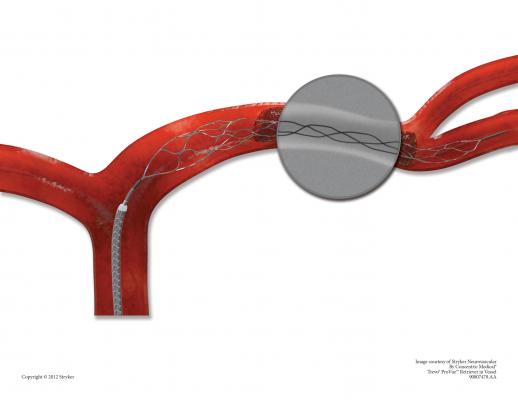
November 19, 2012 — Stryker announced the global commercial launch of the new Trevo ProVue Retriever. The Trevo ProVue Retriever is the first clot removal device fully visible during the procedure for precise positioning within the clot and optimized clot retrieval in patients experiencing acute ischemic stroke. Previous technology only provided visibility to the edges of the device.
"Trevo ProVue Retriever's full visibility provides a significant clinical benefit," said Professor Liebig, M.D., Head of Neuroradiology, University Hospital of Cologne in Cologne, Germany. "I am impressed with how full device visibility provides more information during the procedure to quickly and easily position the device and enables real-time feedback for a new level of confidence to open blood vessels."
Oregon Health and Science University (OHSU) was the institution where the first patient in the United States was treated using the Trevo ProVue Retriever. "It is of great benefit to be able to see how a device interacts with the clot within the vessel, and may be able to demonstrate an underlying stenosis, as it did in the first patient we treated with the device," said Gary Nesbit, M.D., professor of Neuroradiology, Neurology, Neurosurgery and the Dotter Interventional Institute at OHSU. "This gives me better information for clinical decisions and potentially increases the chance for better outcomes for my patients."
"Trevo ProVue sets a new standard in stent retriever technology. It's the first fully visible device allowing physicians to visualize placement, clot interaction and retrieval – that can make a big difference in patient treatment," said Mark Paul, president of Stryker's Neurovascular division.
The Trevo ProVue Retriever is supported by robust clinical evidence from the Trevo family of Retrievers in the TREVO and TREVO 2 clinical trials, which demonstrated high revascularization and improved clinical outcomes compared to the first generation Merci Retriever. The device has been granted 510(k) market clearance by the U.S. Food and Drug Administration (FDA) and is also available in international markets where it has been cleared for sale.
For more information: www.stryker.com


 November 21, 2022
November 21, 2022 




