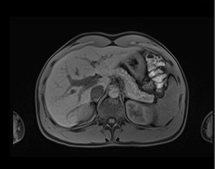
June 4, 2013 — Siemens Healthcare has announced that the U.S. Food and Drug Administration (FDA) recently cleared the company’s CAIPIRINHA (Controlled Aliasing in Volumetric Parallel Imaging Results IN Higher Acceleration) software as part of Siemens’ syngo MR D13A software package for parallel magnetic resonance imaging (MRI). The MRI software helps enable patients with breath-holding difficulties to reduce the amount of time they hold their breath by up to 50 percent without sacrificing imaging resolution or contrast.
Siemens’ CAIPIRINHA software enables acquisition of higher-quality 3-D volumetric interpolated breath-hold sequence (VIBE) T1 images through higher acceleration factors. factors. CAIPIRINHA software is included as standard with Siemens’ D13A software for MAGNETOM Aera 1.5 Tesla, Magnetom Skyra 3.0T, Magnetom Avanto 1.5T, and Magnetom Verio 3.0T systems. It is also available with Siemens’ D14 software for the Magnetom Essenza 1.5T system.
For more information: www.siemens.com/healthcare


 January 21, 2026
January 21, 2026