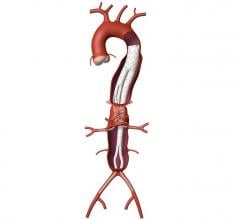
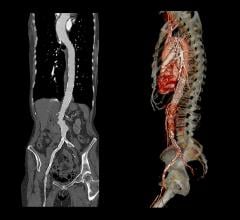
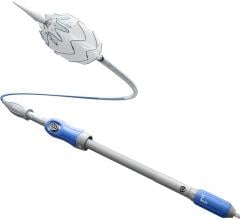
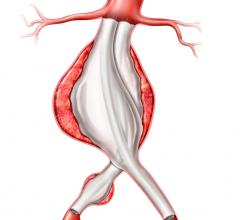
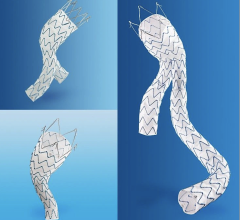
Medtronic's Talent Abdominal Stent Graft is FDA cleared for use on the CoilTrac Delivery System, a medical device that expands patient access to a minimally-invasive treatment option for n Memorial Hospital's Endovascular Program in Baltimore, was the principal investigator for the study.
A Medtronic sponsored study demonstrated that the Talent Abdominal Stent Graft well exceeded the SVS Control in freedom from major adverse events at 30 days, with a statistically significant difference between the two groups: Talent, 89.2 percent; SVS Control, 44.0 percent. Although patients receiving the Talent Abdominal Stent Graft were older and had a higher baseline rate of co-morbidities, at 30 days post-implant they experienced lower rates of major adverse events compared with subjects treated with open surgery. Importantly, there were no aneurysm ruptures and no conversions to open surgery in the Talent group up to 12 months after device implantation.
April 2008

 April 26, 2023
April 26, 2023