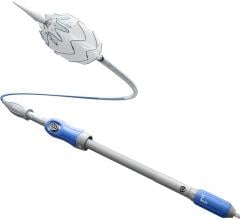
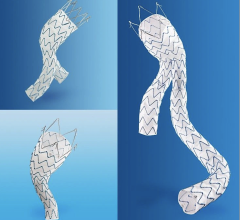
July 1, 2009 – W. L. Gore & Associates said today it received FDA for a manufacturing change to the GORE VIABAHN Endoprosthesis, which is a result of the precision laser trimming technology that enables the removal of excess material at the device margin, resulting in a contoured edge.
The device is the only stent graft approved by the FDA for the treatment of patients suffering from peripheral arterial disease (PAD) in superficial femoral artery (SFA) lesions and iliac artery lesions. In the U.S. alone, as many as 12 million people suffer from PAD.
“I commonly use the GORE VIABAHN Device in my practice for treatment of patients with complex SFA lesions,” said Darren B. Schneider, M.D., associate professor of vascular surgery and radiology at the University of California, San Francisco. “My hope is that the laser contoured edge at the proximal end may improve flow dynamics of blood entering the endoprosthesis.”
The GORE VIABAHN Endoprosthesis is constructed with a durable, reinforced, biocompatible, expanded polytetrafluoroethylene (ePTFE) liner attached to an external nitinol stent structure. The outstanding flexibility of the GORE VIABAHN Endoprothesis enables it to traverse tortuous areas of the SFA and conform to the complex anatomy of the artery. The FDA initially approved the device in 2005 for treating PAD in the SFA. Later in 2007, Gore made modifications, which include reducing the profile and adding a heparin bioactive surface.
GORE VIABAHN Endoprosthesis is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery lesions with reference diameters ranging from 4 mm to 7.5 mm. The GORE VIABAHN Endoprosthesis is indicated for improving blood flow with symptomatic peripheral arterial disease in the iliac artery lesions with reference vessel diameters from 4 mm to 12 mm.
For more information: www.goremedical.com

 April 26, 2023
April 26, 2023