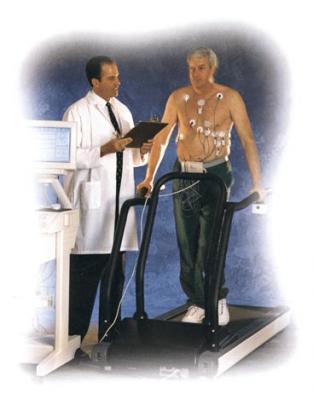
April 19, 2010 – A microvolt T-wave alternans (MTWA) original equipment manufacturer (OEM) module is available to better measure small heartbeat irregularities that indicate a patient’s heightened risk for sudden cardiac arrest. The module allows electrocardiogram (ECG) manufacturers to include the technology in their devices. The U.S. Food and Drug Administration (FDA) gave 510(k) market clearance to the product last week. It allows Cambridge Heart to begin marketing the MTWA OEM module integrated with the Q-Stress line of stress systems manufactured by Cardiac Science Corporation Inc. In June 2009, Cambridge Heart and Cardiac Science entered into a five-year development and distribution partnership, whereby Cardiac Science will market the MTWA OEM module to its existing base of installed stress systems as an upgrade, and to new stress customers as an optional feature. Development of the MTWA OEM module was completed in early February 2010. Sales training and prelaunch activities are taking place between now and an expected launch date later this year. "The development of MTWA as an add-on module for our stress systems allows us to bring new technology to our customers and extend our leadership in the stress segment,” said Dave Marver, Cardiac Science President and CEO. “This initiative is consistent with our goal to help our physician customers by offering simple, cost-effective and efficient devices that improve public health." Cambridge Heart ‘s proprietary microvolt T-wave alternans measurement technologies include the analytic spectral method and very sensitive disposable electrode sensors. The company’s MTWA test, originally based on research conducted at the Massachusetts Institute of Technology, is reimbursed by Medicare under its National Coverage Policy. For more information: www.cambridgeheart.com


 January 15, 2026
January 15, 2026 









