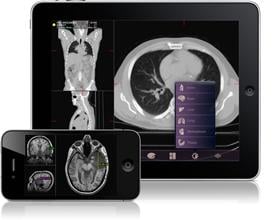
February 4, 2011 – A new mobile radiology application cleared today by the U.S. Food and Drug Administration (FDA) will allow physicians to view and make a diagnosis from medical images on the Apple iPhone and iPad. The application is the first cleared by the FDA for viewing images and making medical diagnoses based on computed tomography (CT), magnetic resonance imaging (MRI) and nuclear medicine technology, such as positron emission tomography (PET).
The FDA said the application is not intended to replace full workstations and is indicated for use only when there is no access to a workstation. However, the application’s clearance will likely be the first of many, as physicians are rapidly adopting iPads as a replacement for traditional clipboards. The device offers a mobile workstation to access and enter information for patient records, images and test results. At the Radiological Society of North America (RSNA) 2010 meeting in December, the event was unofficially dubbed “the year of the iPad” due to the vast number of vendors who were showing new iPad applications to access and manipulate radiology images.
“This important mobile technology provides physicians with the ability to immediately view images and make diagnoses without having to be back at the workstation or wait for film,” said William Maisel, M.D., MPH, chief scientist and deputy director for science in the FDA’s Center for Devices and Radiological Health.
Radiology images taken in the hospital or physician’s office are compressed for secure network transfer, then sent to the appropriate portable wireless device via software called Mobile MIM. Mobile MIM, manufactured by Cleveland-based MIM Software Inc., allows the physician to measure distance on the image and image intensity values and display measurement lines, annotations and regions of interest.
In its evaluation, the FDA reviewed performance test results on various portable devices. These tests measured luminance, image quality (resolution) and noise in accordance with international standards and guidelines. The FDA also reviewed results from demonstration studies with qualified radiologists under different lighting conditions. In an FDA-issued press release, it said all participants agreed that the device was sufficient for diagnostic image interpretation under the recommended lighting conditions.
The display performance of mobile devices can experience significant variations in luminance levels even between mobile devices of the same model. The Mobile MIM application includes sufficient labeling and safety features to mitigate the risk of poor image display due to improper screen luminance or lighting conditions. The device includes an interactive contrast test in which a small part of the screen is a slightly different shade than the rest of the screen. If the physician can identify and tap this portion of the screen, then the lighting conditions are not interfering with his or her ability to discern subtle differences in contrast. In addition, a safety guide is included within the application.
Outside of the United States, Mobile MIM is available in 14 languages.
For more information: www.mimsoftware.com


 December 17, 2015
December 17, 2015 

