Technology | December 30, 2013
Digisonics, Medis Partner to Provide Cardiovascular MRI Review, Analysis, Structured Reporting Solution
December 30, 2013 — Digisonics and Medis have partnered to provide a single system solution for image analysis and structured reporting of pediatric and adult cardiovascular magnetic resonance (MR) studies. The integrated system solution between Digisonics and Medis will result in a more efficient MR workflow.
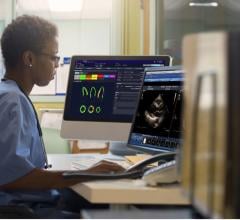
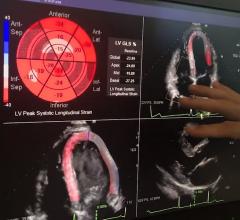
Users will have access to fast image review and analysis of their MR studies via Medis QMass ES advanced quantitation software, which provides built-in, disease-oriented review layouts and cross referencing tools. Users can analyze cardiovascular dimensions and morphology, myocardial function (MR and computed tomography [CT]), phase-contrast blood flow, tissue characterization (late enhancement, T2 weighted edema, T2/T2 mapping, T1 mapping), myocardial perfusion and MR angiography. Results from the analysis completed in Medis will be saved directly back into the Digisonics cardiovascular information system.
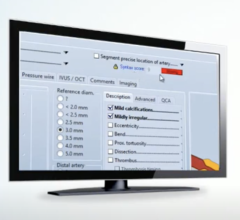
With the completed image analysis from Medis, clinicians can summarize their findings in a structured report using Digisonics’ picklists and summary macros. These concise (usually one page) and highly configurable reports also offer the ability to embed post-processed study images and graphics such as charts or clinical diagrams.
QMass is cleared for market in the United States. The use of aforementioned contrast agents for cardiovascular MR procedures is not U.S. Food and Drug Administration (FDA)-approved; the myocardial perfusion and tissue characterization modules of QMass have not received FDA 510(k) market clearance. Myocardial perfusion and tissue characterization modules are available on demand in the United States for research purpose only.
For more information: www.digisonics.com, www.medis.nl


 November 06, 2025
November 06, 2025