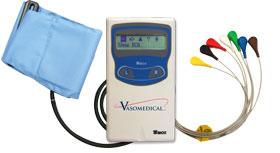
April 13, 2010 – The U.S. Food and Drug Administration (FDA) recently cleared a combined electrocardiogram (ECG) Holter and ambulatory blood pressure monitoring recorder and software analysis system.
The Vasomedical-Biox Model 2301 system is among the first combined systems to simultaneously and continuously record and store ECG and blood pressure data for 24 hours. The ability to view these parameters simultaneously, side by side gives the physician more useful information to evaluate the patient's cardiovascular status.
In addition to being a compact device that replaces two separate recorders, it also provides interactive blood pressure recording at times of certain cardiac abnormalities. This can enhance the diagnostic value of the recorded data, while increasing patient comfort and ease of use.
For more information: www.vasomedical.com


 February 14, 2022
February 14, 2022 




