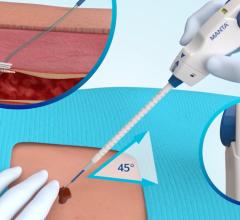
August 18, 2009 – Cardiva Medical Inc. recently received FDA clearance for its Cardiva Catalyst III, which the company says is the first drug-coated vascular closure device for the intervention cardiology market.
The device will be highlighted by Cardiva at TCT 2009 Sept. 22-25. The Catalyst III is coated with protamine sulfate, a drug that neutralizes heparin in the tissue adjacent to the device. This local heparin reversal facilitates quick and efficient vessel closure as an adjunct to manual compression in patients undergoing anticoagulation with heparin. Catalyst III’s protamine coating contacts the tissue tract from the arteriotomy site to the point of percutaneous entry in the skin.
Cardiva estimates that annually 1.7 million patients in the United States receive heparin during an endovascular procedure; the majority of these cases are for peripheral vascular disease, the fastest growing segment in the percutaneous procedure market.
Initial use of the Catalyst III system took place in the Cardiovascular Institute of the South located at Terrebonne General Medical Center in Houma, LA., under the direction of its founder, interventional cardiologist, Craig Walker, M.D.
“We believe that localized protamine in the tissue tract makes a big difference,” Dr. Walker said. “Not only can we accomplish rapid, natural vessel closure for our patients, but we can also improve our efficiency while utilizing a more cost-effective and time-tested anticoagulant.”
For more information: www.cardivamedical.com


 October 07, 2025
October 07, 2025