May 7, 2014 — Cardiotek B.V., a subsidiary of Schwarzer, a privately held medical device company focused on electrophysiologic (EP) and hemodynamic measurement systems, today announced it will commence commercialization of its EP-Tracer system following the 2014 Heart Rhythm Society (HRS) annual scientific sessions in San Francisco.
The EP-Tracer system is a recording device with a built-in dual-channel programmable stimulator that is currently used worldwide. Targeted U.S. commercialization of the U.S. Food and Drug Administration (FDA) approved EP-Tracer system will begin later this month as part of a comprehensive U.S. go-to-market strategy.
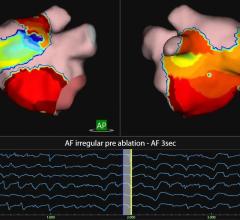
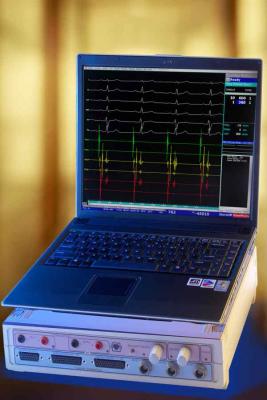
The EP-Tracer system is an EP measurement system used to acquire, filter, digitize, amplify, display and record signals obtained during electrophysiological studies and related procedures. The compact, easy-to-use recording system provides superior signal strength with a built-in dual channel programmable stimulator for EP studies. The EP-Tracer system is commercially available in Europe with approximately 1,000 systems installed worldwide.
The EP-Tracer system provides signal quality controlled by a compact, single-user unit. The integrated programmable stimulator, which allows stimulation on all intracardiac inputs, has three model options and offers up to 102 intracardiac channels. System customization allows for portable, mobile and stationary configurations to optimally suit the customer needs and improve system utilization across EP labs.
"Throughout the majority of my career and for thousands of ablations, I have used the EP-Tracer system as my primary EP recording system. The system offers superior signal quality in a compact, single-user system," said Josep Brugada M.D. Ph.D., electrophysiologist and medical director at the University Hospital Clinic of Barcelona in Spain. "The EP-Tracer system provides a simplified approach to therapeutic procedures."
EP studies are used to diagnose and treat a variety of conditions in which the heart beats abnormally, including atrial fibrillation (AF). During EP testing, the recording system acquires, measures and records intracardiac signals as well as stimulates the heart to assist the physician in the diagnosis and treatment.
"The EP-Tracer system is one of the preferred recording devices in Europe with an installed base of approximately 1,000 systems," said Frank-P. Klein, president and chief commercial officer of Schwarzer. "We are excited to bring the EP-Tracer system to the United States and believe it will transform the market by offering physicians a higher quality and more portable system for EP procedures."
The EP-Tracer system received 510(k) clearance in March 2014.
For more information: www.cardiotek.com


 September 05, 2025
September 05, 2025