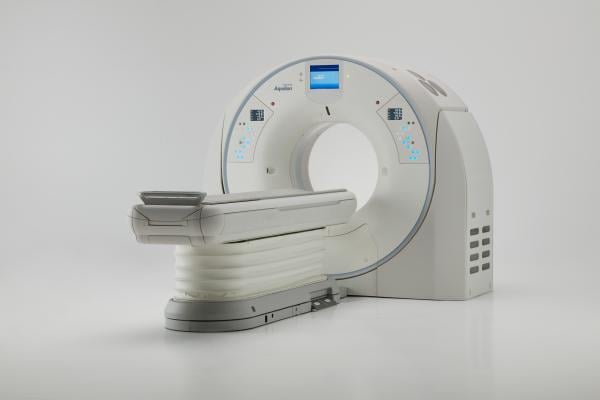
April 13, 2018 — Canon Medical Systems USA Inc. this week received U.S. Food and Drug Administration (FDA) clearance for the Aquilion Precision system, which it calls the world’s first Ultra-High Resolution computed tomography (UHR CT) system. The system can resolve anatomy as small as 150 microns and is designed to provide more than twice the resolution, typically seen only in cath labs. Containing an all-new detector as well as tube, gantry and reconstruction technologies, the system may make it possible to help expand visualization of disease thanks to new features that offer improved image detail.
The Aquilion Precision CT system emphasizes dose efficiency with detector channels that are only 0.25 mm thick. This, combined with substantial improvements in scintillator quantum efficiency, detector circuitry and other DAS components, results in a dose-efficient detector with ultra-high resolution capabilities. The system features resolution never before seen in CT imaging, according to Canon, with what it calls the industry’s smallest Focal Spot Tube at 0.4 mm x 0.5 mm and the industry’s first routine 1024 x 1024 Reconstruction Matrix.
For more information: www.us.medical.canon


 January 28, 2026
January 28, 2026 









