Feb. 11, 2025 — UltraSight, a company committed to enhancing the efficiency and productivity of cardiac ultrasound, recently announced support from Bristol Myers Squibb (BMS) for a study that aims to improve access to echocardiographic assessments for patients with obstructive hypertrophic cardiomyopathy (oHCM).
Cardiac ultrasound is routinely used as an essential tool in the diagnosis and management of oHCM.
“UltraSight was founded with the goal of giving clinicians the ability to scan patients, wherever patients may be,” said Davidi Vortman, CEO of UltraSight. “By applying the power of machine learning to imaging, our aim is to streamline patient cardiac workflows with UltraSight real-time guidance for better, more efficient, and effective care. We are thrilled to receive support from BMS on this study with the goal of evaluating how this technology could help patients in need of consistent and reliable cardiac follow-up.”
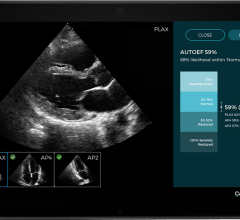
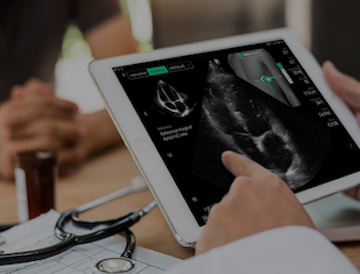
The study will be launched at Cleveland Clinic and will focus on oHCM patients with the aim to validate the quality and accuracy of the monitoring echocardiograms performed by non-sonographers using UltraSight’s Real-Time Guidance software.
UltraSight’s Real-Time Guidance software provides real-time assistance for healthcare providers, guiding them to acquire diagnostic-quality echocardiography images. This approach is designed to optimize and streamline cardiac monitoring and other clinical workflows, enabling non-sonographers to achieve consistent, diagnostic-quality ultrasound thus improving patient access, operational efficiency, and patient care. By putting cardiac ultrasound in the hands of any medical professional, UltraSight’s software can help expand access to care, bringing cardiac ultrasound to more hospitals and cardiology clinics across the country.
“At Bristol Myers Squibb, we are committed to forging the path to improve cardiac care through innovative science,” said Cecilia Marta, Vice President, US Medical Cardiovascular. “This support of UltraSight exemplifies our commitment to transforming patients’ lives through science by supporting cutting edge research.
Recruitment for the trial will begin in 2025. Please check https://clinicaltrials.gov/ for updates.
For more information about UltraSight, visit https://ultrasight.com/. For more information about Bristol Myers Squibb, visit https://www.bms.com/.


 October 09, 2025
October 09, 2025