June 7, 2024 — BioCardia, Inc., a company focused on cellular and cell-derived therapeutics for the treatment of cardiovascular and pulmonary diseases, announced that the Unites States Patent Office has granted Patent No: 11,986,611 titled “Radial and Transendocardial Delivery Catheter,” with a patent term that will expire in 2036.
The present invention relates to medical methods and systems suitable for substance delivery to the heart via a radial artery and for the intracardiac delivery of cellular aggregates and other agglomerated materials.
Radial artery delivery is a means by which cardiac catheters are advanced through a blood vessel in a patient’s wrist to treat the heart. This approach has significant advantages for patients, enabling them to leave the hospital soon after the procedure with a band aid on their wrist and their arm in a simple sling, allowing them to immediately return to their active lives. In addition, a radial approach permits hospitals and other care centers to greatly reduce costs by eliminating the need for an overnight stay by the patient. For these reasons, trans-radial access is becoming the default approach in many cardiac centers worldwide. Enabling radial access has enormous potential advantages for biotherapeutic delivery to the heart.
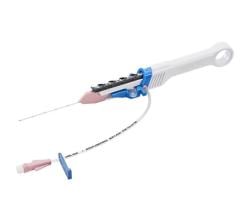
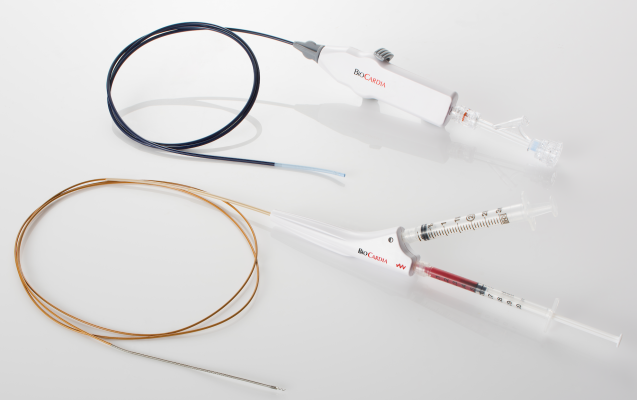
BioCardia’s Helix system, used in our ongoing clinical trials, is the only known system with patented designs that can enable radial transendocardial biotherapeutic delivery. The issued patent claims further protect this approach and add value to both BioCardia’s therapeutic programs and those of our biotherapeutic delivery partners.
Therapeutic cell aggregates have the potential advantage over single cell suspensions by enhancing retention in the heart to maximize therapeutic benefit. However, the delivery of cell aggregates carries greater risks of potentially life-threatening strokes should they leak into the ventricular chamber. As 20% of the blood in the heart chamber goes to the brain, 20% of any cell or cell aggregates released in the heart are expected to obstruct the first cerebral artery that is too small to allow the cell or cell aggregate to pass. The larger the cell aggregate, the larger the vessel they are capable of obstructing, with greater risk of a significant life-threatening stroke.
BioCardia’s delivery systems are designed to prevent leakage of therapeutic agents into the ventricular chamber by providing stable and safe engagement to the heart tissue during the delivery process. The issued patent claims further protect this design and add value to both BioCardia’s therapeutic programs and those of our biotherapeutic delivery partners.
“Our minimally invasive biotherapeutic delivery platforms enable the successful development of cell and gene-based therapies for the heart,” said Dr. Peter Altman, BioCardia CEO. “The Helix platform underlies BioCardia’s cell therapy clinical programs and this recent patent issuance provides additional protection to our technology and product offerings in the United States for at least another dozen years. This is just one of many patent applications we are advancing to protect our value creation for the benefit of shareholders, which in turn enables these advances to be supported for expected benefit to millions of patients.”
For more information: www.biocardia.com


 January 29, 2026
January 29, 2026