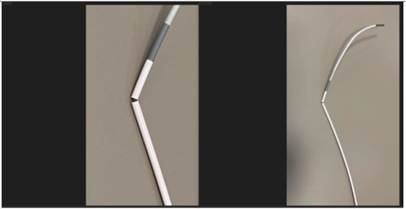
April 4, 2024 — The U.S. Food and Drug Administration (FDA) announced that Medos International Sàrl is recalling Cerenovus CEREBASE DA Guide Sheath due to a crack found in the far end of the catheter, where different parts of the catheter are joined together.
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
Recalled Product
- Product Names:
- CERENOVUS CEREBASE DA Guide Sheath, Part Numbers:
- GS9070SD;
- GS9080SD;
- GS9090SD;
- GS9095SD;
- Neurovascular guide catheter, single-use
- CERENOVUS CEREBASE DA Guide Sheath, Part Numbers:
- Product Codes: QJP
- Manufacturing Dates: July 7, 2023, and later
- Distribution Dates: June 14, 2023, to December 14, 2023
- Devices Recalled in the U.S.: 1,343
- Date Initiated by Firm: February 2, 2024
Device Use
The CEREBASE DA Guide Sheath is a neurovascular catheter. This type of catheter is commonly used in procedures where precise navigation and access to blood vessels in the brain are required. This catheter is designed to help deliver interventional devices into the blood vessels in the brain, allowing doctors to place devices like stents or coils, to treat neurovascular diseases and conditions.
Reason for Recall
Medos International Sàrl is recalling the Cerenovus CEREBASE DA Guide Sheath due to complaints describing fractures of the distal catheter shaft. This is a crack found in the far end of the catheter that is placed in the vessels in the brain, where different parts of the catheter are joined together.
The use of the affected product may result in surgical procedural delay, vascular injury or hemorrhage, and in extreme rare occasions it may result in embolism.
There have been 3 reported injuries.
There have been no reports of death.
Who May be Affected
- Healthcare professionals who use the CEREBASE DA Guide Sheath in vascular procedures such as surgeries on the brain.
- People who are having procedures where precise navigation and access to blood vessels in the brain are required.
What to Do
On February 2, 2024, Johnson & Johnson MedTech, on behalf of Medos, sent all affected customers an Urgent Medical Device Recall.
The letter requested customers to:
- Examine inventory and quarantine any product subject to this recall.
- Remove any product subject to this recall and communicate the issue to anyone who needs to be informed.
- Contact any facility where affected products may have been forwarded to arrange their return.
- Complete the Business Reply Form (BRF) confirming receipt of the notice and scan and email signed form to [email protected] or fax it to (305) 265-6889.
- Attention Line: Cerebase FA2350411
- Follow instructions in the letter and immediately call 1-844-483-3882 to return any inventory of CEREBASE DA Guide Sheath devices subject to the recall. To receive credit reimbursement, customers must return product subject to the recall.
- Attention Line: Cerebase FA2350411
Contact Information
Customers in the U.S. with questions about this recall should contact Medos at [email protected]
Additional Resources
How do I report a problem?
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program using an online form, regular mail, or FAX.


 January 15, 2026
January 15, 2026 









