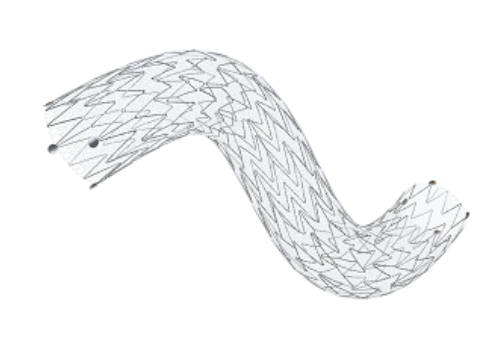
November 29, 2023 — InspireMD, Inc., developer of the CGuard Embolic Prevention Stent System (EPS) for the prevention of stroke, today announced that the company has entered into a strategic agreement with the Jacobs Institute at the State University of New York at Buffalo, and Dr. Adnan Siddiqui, Vice-Chairman and Professor of Neurosurgery, to execute an Early Feasibility Study (EFS) evaluating the CGuard EPS carotid stent to treat severe carotid stenosis or occlusion, in conjunction with thrombectomy, in patients presenting with acute ischemic stroke and tandem lesions.
Marvin Slosman, chief executive officer of InspireMD, stated, “This Early Feasibility Study provides the appropriate platform for our investment in this indication to address Carotid lesions in acute stroke settings and reinforces our commitment to the neuro community, as we aim to demonstrate that CGuard EPS is optimally designed with a low metal surface and MicroNet mesh covering for superior embolic protection during these acute events. We look forward to results from this study and view the tandem lesion indication as a critical component of our long-term growth strategy for the CGuard stent platform.”
Dr. Adnan Siddiqui added, “Tandem strokes with occlusion of both cervical and intracranial vessels are very common (~20%). To date, all clinical trials conducted in the U.S. for acute stroke from large vessel occlusions have specifically excluded them, resulting in a lack of guidelines and resultant great variability in the management of these lesions. As a result, there are currently no FDA approved stents for this specific indication. The most dramatic part is that trials conducted outside the U.S. have shown the value of thrombectomy in this patient population is the most beneficial of any large vessel occlusion category. Retrospective analyses from large centers across the globe have suggested the safety and efficacy of stenting in conjunction with intracranial thrombectomy. There is strong evidence to suggest that stenting in these lesions is superior to not stenting.”
“I am delighted that InspireMD, with its C-Guard device, which has low metal surface area and therefore reduces thrombotic risk, as well as a MicroNet mesh that protects plaque prolapse intra- and post- procedure, have elected to investigate this critical indication. I am also excited that this brings the carotid disease as it pertains to neurointerventionalists to the forefront. This EFS will help lead to a design of the pivotal trial that can bring this large, neglected population into the standard practice of revascularization for stroke,” Dr. Siddiqui concluded.
The EFS is expected to enroll 15 patients across three U.S. trial sites and explore the safety and feasibility of using the CGuard EPS carotid stent, with its unique MicroNet™ mesh covering, to treat acute ischemic stroke patients with tandem lesions.
For more information: www.inspiremd.com


 January 05, 2026
January 05, 2026 









